Treatment Gaps in Advanced AF
AF is a global health problem, affecting between 2% and 4% of adults worldwide and is increasing.1 Based on the Global Burden of Disease, Injuries and Risk Factors Study, an estimated 59.7 million people globally had prevalent AF or atrial flutter (AFL) in 2019 and approximately 300,000 deaths were attributed to AF/AFL.2 Three primary concerns with unmanaged AF are an increased risk of stroke, progressive or worsening heart failure and increased mortality. Other less acute but nonetheless important implications of AF include impaired patient quality-of-life, increased healthcare resource use and potential for cognitive decline. It is estimated that the AF-related cost to the healthcare system in the US is $6 billion per year for AF alone and up to $26 billion including other cardiovascular and non-cardiovascular costs.3
Anti-arrhythmic drugs (AADs) and ablation are key treatment strategies for treating AF, and recent evidence has suggested that early rhythm control with these strategies is more beneficial than traditional care focused on rate control.4 The prioritisation and success of ablative treatment strategies can be influenced by the type of AF. Paroxysmal AF accounts for approximately 30% of AF cases, whereas non-paroxysmal AF – including persistent and long-standing persistent AF – account for the majority at approximately 70%.5 Treatment of paroxysmal AF is based on pulmonary vein isolation (PVI), which is considered the cornerstone of AF treatment. Randomised clinical trial data support favourable effectiveness of endocardial radiofrequency (RF) or cryoballoon ablation for paroxysmal AF.6–8 These findings are complemented by long-term outcomes in catheter ablation registries.9 However, non-randomised clinical trial data exist for catheter ablation of persistent and long-standing persistent AF. Two recent contemporary endocardial ablation trials, STOP PERSISTENT AF and PRECEPT, were non-randomised and included patients with less than 6 months or 12 months of persistent AF.10,11 Ablation was primarily focused on PVI, although 56% of patients in the PRECEPT trial had additional linear and/or complex fractionated atrial electrogram (CFAE) ablation.11 Treatment gaps for persistent and long-standing persistent AF include the role of systematic non-PVI ablation in improving clinical outcomes in persistent AF and an effective treatment strategy for long-standing persistent AF. In this review, we explore both topics in context of hybrid epicardial-endocardial ablation and specifically recent results of the prospective, randomised, multi-centre CONVERGE clinical trial.
Role for Posterior Wall Ablation and Current Challenges
AF is initiated by a focal arrhythmogenic trigger. Seminal work by Haissaguerre et al. first demonstrated ectopic beats within the pulmonary veins (PVs) in AF, thereby establishing the PVs as the main target for AF ablation strategies.12 To this day, PVI is still considered the cornerstone of AF treatment and it is certainly mandatory for any ablation strategy to treat AF. However, it is also recognised that non-PV triggers of AF are present, even in paroxysmal AF or groups of unselected AF.13,14 Regions of AF triggers include the left atrial posterior wall, the left atrial appendage (LAA), coronary sinus, superior vena cava, crista terminalis, and ligament of Marshall. Given the diminished success rates of PVI-only strategies as AF progresses, it is reasonable to assume that contribution of non-PV triggers is increased in persistent and long-standing persistent AF relative to paroxysmal AF.
The posterior wall of the left atrium has been a major focus of extra-PV ablation. The posterior wall shares embryological origin with the PVs, as the left atrium incorporates parts of the embryological common PV after its second bifurcation.15,16 Therefore, the intrinsic propensity of PV cardiomyocytes for arrhythmogenicity are shared with those of the posterior wall.14 In addition to the presence of triggers, the posterior wall also acts as anatomical substrate for AF thus it can initiate and potentiate AF as the disease continues and progresses. Like the unique electrophysiological properties of its cardiomyocytes, the posterior wall also has inherent anatomical features that become substrate for AF. These include the heterogeneous orientation of myocardial fibres, tissue thickness variation, presence of epicardial fat and density of autonomic innervation and ganglionated plexi.17 Additionally, the posterior wall is subject to electroanatomical changes that occur with continuous AF, including development of fibrosis and infiltration of fat into the myocardial tissue, which can contribute to abnormal conduction.18 Myocytes in the posterior wall tend to have shorter action potential duration than the rest of the atrium and this enhances the tendency to arrhythmogenesis.
Isolation of the posterior wall including PVI has remained a constant through iterations of the Cox-Maze procedure. Voeller et al. showed improved freedom from atrial arrhythmias off AADs with the inclusion of both a roof and floor line connecting PVI lines (i.e. box lesion) to isolate the entire posterior wall compared to only a roof line connecting PVI lines.19 Several strategies have been described to isolate the posterior wall with endocardial catheters, including a single-ring encompassing the PVs and posterior wall, a box lesion and debulking the entire posterior wall.20 There are several potential impediments to successful posterior wall isolation using endocardial ablation only. The durability of endocardial posterior wall isolation after a single procedure is suboptimal with a recent meta-analysis reporting a 63% rate of posterior wall reconnection.21–23 Technological, anatomical and functional factors may contribute to this issue.24 There are still limitations to catheter-based technology to make continuous linear lesions without the potential for pro-arrhythmic gaps. Endocardial ablation of the posterior wall carries some risk of collateral damage with the close proximity of the oesophagus to the posterior left atrium, as well as the phrenic nerve and lungs. Mitigation strategies may reduce the potential for thermal injury but also may reduce the likelihood of successful, durable posterior wall isolation. In addition, endocardial-epicardial dissociation – asynchrony in the activation patterns of the endocardial and epicardial surfaces – may increase as AF progresses to persistent and long-standing persistent form.25–27 Endocardial-only or epicardial-only ablation may not be sufficient to overcome this observed dissociation.
Meta-analyses have concluded that overall there may be a benefit of posterior wall isolation in addition to PVI in persistent and long-standing persistent AF.23,28 However, outcomes with endocardial posterior wall ablation are variable across studies, particularly in randomised clinical trials comparing endocardial PVI to endocardial PVI with posterior wall ablation.21,29–33 The mixed outcomes that have been reported are likely a product of the various endocardial strategies to address the posterior wall, lack of routine verification of posterior wall isolation and inherent limitations of endocardial ablation to overcome complex atrial substrate that is present in persistent and long-standing persistent AF.
Hybrid Convergent Approach
Hybrid epicardial-endocardial ablation is an ablation strategy aimed at isolating the PVs and left atrial posterior wall using a heart team collaboration between cardiothoracic surgery and electrophysiology and combined techniques from both disciplines. Epicardial ablation is performed first, via a minimally invasive surgical approach, which is then followed by endocardial mapping and ablation to complete PVI as well as to address any identified gaps or arrhythmogenic areas. There are currently two predominant hybrid ablation approaches. A thoracoscopic hybrid ablation technique uses bipolar RF clamps and pens for epicardial ablation followed by endocardial catheter ablation.34 Randomised clinical trial results from the HARTCAP-AF trial were recently reported, showing significantly improved arrhythmia-free survival without AADs at 12 months with thoracoscopic hybrid ablation compared to endocardial ablation.35 Other randomised clinical trials comparing thoracoscopic hybrid ablation and endocardial ablation are on-going.
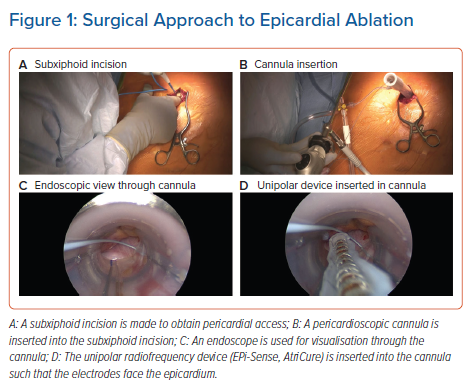
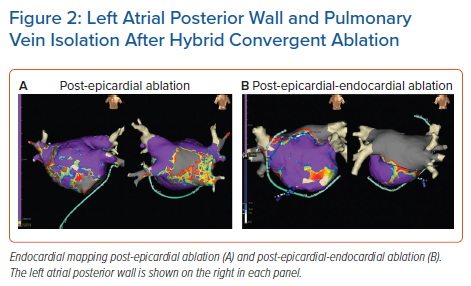
The other epicardial-endocardial approach to ablation and main focus of this review is the hybrid convergent procedure. In this technique, access to the pericardial space is achieved through a subxiphoid incision (Figure 1). Detailed technical aspects of the procedure and best practices have been described elsewhere and are reviewed briefly here.24,36,37 Using endoscopic visualisation, an irrigated, unipolar RF catheter is used to make parallel rows of ablation lines across the left atrial posterior wall between the PVs. The catheter is designed such that the electrodes face the epicardium during ablation so that energy is applied towards the heart, in contrast with endocardial energy application that is applied from the inside of the heart towards the outside. The superior epicardial ablation margin is naturally limited by the pericardial reflections, which are not dissected, and the inferior margin is created such that the area between the inferior PVs and the coronary sinus is not ablated to avoid creating left AFL. Following surgical closure, endocardial mapping and ablation are performed to complete the lesion set so that both the PVs and posterior wall are electrically isolated at the end of the procedure (Figure 2). Both the epicardial and endocardial procedures can be performed on the same day, and even in the same room if set up as a hybrid room. Alternatively, the procedures can be staged at least one month apart. The pre- and post-procedure workflows and coordination of the surgical-electrophysiological heart team require considerable attention particularly when setting up a new programme, but represent a unique opportunity for multidisciplinary arrhythmia management.24 The electrophysiologist plays a key role in coordinating collaboration with surgical, administrative, and nursing colleagues in this process.
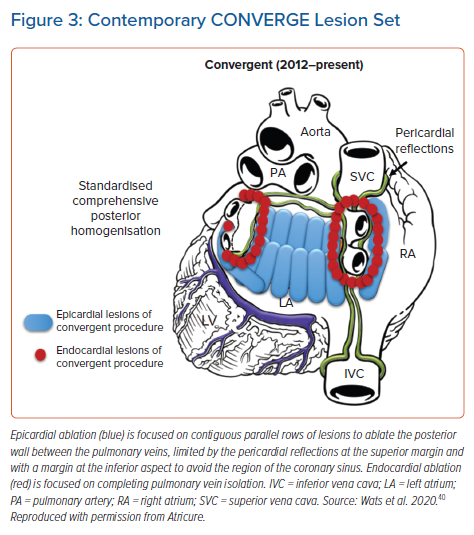
A previous meta-analysis comparing hybrid ablation studies with epicardial-only surgical ablation reported similar clinical success rates between the methods, but higher complications with hybrid ablation.38 This meta-analysis combined six studies that used hybrid thoracoscopic ablation and three studies that used hybrid convergent ablation. The majority of the convergent procedures were performed between 2009 and 2013 using earlier device technology and lesion sets. With gained experience, the hybrid convergent procedure has evolved over time since its original development in 2009, with many of these changes focused on reducing the risk of complications.39 The lesion set has evolved from an extra-cardiac Maze lesion set to a posterior wall box to finally a contiguous set of parallel lesions across the posterior wall (Figure 3).40 Other changes to the procedure include new generations of the unipolar RF device and pericardial access approach from transdiaphragmatic to subxiphoid. Additionally, peri-procedural medication strategies have evolved such that many institutions use an uninterrupted anticoagulation strategy prior to the epicardial procedure to mitigate risk of thromboembolic events and prophylactically prescribe anti-inflammatory drugs to prevent delayed inflammatory pericardial effusions.36
As previously mentioned, thermal injury to surrounding tissues including the oesophagus is a concern with any posterior wall ablation. As identified in the meta-analysis by Pearman et al., atrio-oesophageal fistulas were reported with early convergent procedure experience.38 Several procedural steps are now recommended to avoid thermal injury to non-target tissue during epicardial ablation. The unipolar RF device has electrodes that are exposed on one surface only; this surface is oriented towards, and in direct contact with, the heart. Although the other surface abuts the pericardium, this surface is unexposed and well-insulated, providing very good protection against collateral thermal injury to the pericardium and adjacent non-cardiac structures such as the oesophagus. The device also has an internal perfusion system for cooling. The device instructions for use and best practices recommend use of oesophageal temperature monitoring to avoid increases >0.5°C or absolute temperature >38°C during ablation.36 Room temperature saline irrigation through the cannula also helps reduce heating within the pericardial space. It is also recommended to not proceed with ablation if overlapping anatomical structures cannot be separated and thermally isolated.
The CONVERGE Clinical Trial
The epicardial-endocardial hybrid convergent procedure was compared to endocardial catheter ablation in a prospective, multicentre, randomised clinical trial, Convergence of Epicardial and Endocardial RF Ablation of the Treatment of Symptomatic Persistent AF (CONVERGE; NCT01984346).41,42 The aim of the CONVERGE trial was to evaluate the safety of the Hybrid Convergent procedure and compare its effectiveness with endocardial catheter ablation for the treatment of persistent and long-standing persistent AF. A total of 153 patients with persistent or long-standing persistent AF were randomised 2:1 to either the Hybrid Convergent procedure or endocardial catheter ablation. Forty-two percent of patients in the study had long-standing persistent AF, which was a unique aspect of CONVERGE among contemporary ablation studies to include a substantial proportion of long-standing persistent AF patients. The Hybrid Convergent arm lesion set included epicardial ablation with a unipolar RF catheter focused on the posterior wall and PVs followed by endocardial catheter ablation to complete PVI and address remaining gaps. The catheter ablation arm lesion set was made using an irrigated RF catheter to isolate the PVs and create atrial roof and cavotriscupid isthmus lines. Additional CFAEs were left to operator decision and ultimately 26% of patients in the catheter ablation arm received CFAEs.42 This was considered the optimal endocardial lesion set at the time of study design for persistent AF ablation. Unlike the Hybrid Convergent arm, posterior wall isolation was not performed in the endocardial catheter ablation arm. This was due to several aforementioned factors, which are also reflected in guideline recommendations: inconsistent effectiveness outcomes reported for endocardial posterior wall ablation, concerns for thermal injury or perforation, and substantial reconnection rates that calls into question lesion durability.21–23,29
The primary safety events in the Hybrid Convergent arm included eight major adverse events (7.8%) within 30 days of the procedure, which was less than the pre-specified primary safety goal.42 Four of the events were delayed inflammatory pericardial effusions that resolved without sequalae after planned intervention for pericardial effusion drainage. The primary effectiveness endpoint – freedom from AF/AFL/atrial tachycardia (AT) absent new AADS or increased dose of previously failed AADs – was achieved in 67.7% of patients in the Hybrid Convergent arm compared to 50% in the catheter ablation group (p=0.036). The success rate was also significantly higher in the Hybrid Convergent arm off all AADs (53.5% versus 32.0%; p=0.013) and irrespective of AADs (76.8% versus 60.0%; p=0.033). In effect, the CONVERGE trial met its primary and safety endpoints, showing improved effectiveness with hybrid ablation compared to endocardial catheter ablation.
Other Studies Comparing Hybrid Ablation to Endocardial Ablation
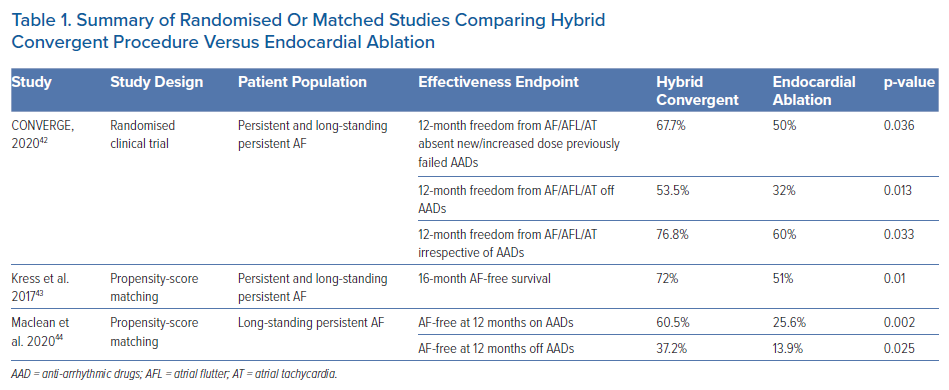
CONVERGE is the only randomised study of hybrid convergent ablation compared to endocardial ablation in persistent and long-standing persistent AF. However, other retrospective analyses that compared hybrid convergent ablation to endocardial catheter ablation have been published (Table 1). Contemporary published experience with similar approach to convergent ablation is consistent with that of CONVERGE, where improved rhythm outcomes were observed with the hybrid convergent procedure compared to endocardial ablation. Kress et al. reported outcomes of patients who received hybrid convergent ablation (using a previous generation unipolar RF catheter for epicardial ablation) compared to endocardial ablation, using propensity-score matching to balance the two groups of 40 patients each.43 Significantly improved rates of arrhythmia recurrence (36.5% versus 57.9%; p=0.013) and freedom from AF (72% versus 51%; p=0.01) at 16 months were achieved in the hybrid convergent group compared to endocardial ablation group, respectively.
In a more recent study, Maclean et al. compared outcomes of 43 patients with exclusively long-standing persistent AF who received hybrid convergent procedure with 43 propensity-matched patients who received endocardial catheter ablation during the same time period between 2013 and 2018.44 At 12 months, AF-free survival was 60.5% with hybrid convergent ablation versus 25.6% (p=0.002) after a single-procedure. Off AADs, 12-month AF-free survival rates were significantly higher in the hybrid convergent group at 37.2% compared to 13.9% (p=0.025) in the endocardial ablation group. The study by Kress et al. did not find a significant difference in procedural complications between groups before matching, while Maclean et al. found a higher overall complication rate in the hybrid convergent group compared to catheter ablation.43,44 Other differences in the studies were that Maclean et al. performed staged hybrid procedures and used exclusively endocardial RF, whereas Kress et al. performed hybrid ablation in the same setting with most patients receiving endocardial cryoballoon. Despite these differences, overall these non-randomised findings are consistent with improved clinical success of hybrid convergent ablation compared to endocardial catheter ablation. A limitation of these studies is that the endocardial approach in the control groups was not strictly defined.
Arrhythmia Burden
By definition, a 30-second atrial arrhythmia recurrence during follow-up resulted in a failure of the primary effectiveness endpoint of CONVERGE and other recent contemporary ablation trials.10,11,42 The 30-second atrial arrhythmia recurrence definition is controversial for its stringency, being outside the limit of detection of many continuous monitoring methods, and the potential to overlook meaningful improvements in symptoms and quality-of-life for patients – particularly those with advanced AF.45 For this reason, AF burden – often defined as the proportion of time a patient is in AF – has been proposed as an appropriate outcome endpoint for persistent and long-standing persistent AF.46 Assessment of AF burden may help shift the overly simplistic perception of AF as a binary disease.47 The CONVERGE trial assessed the reduction of AF burden at 12 months (with 24-hour Holter) and 18 months (with 7-day Holter) following the Hybrid Convergent and catheter ablation procedures. At 12 months, 80% of patients in the Hybrid Convergent arm achieved ≥90% AF burden reduction compared to baseline versus 56.8% of patients in the catheter ablation group (p=0.007). At 18 months, ≥90% AF burden reduction was seen in 74% of patients in the Hybrid Convergent arm compared to 55% in the catheter ablation group (p=0.0395). These results attest to the durability of the Hybrid Convergent procedure compared to endocardial ablation.
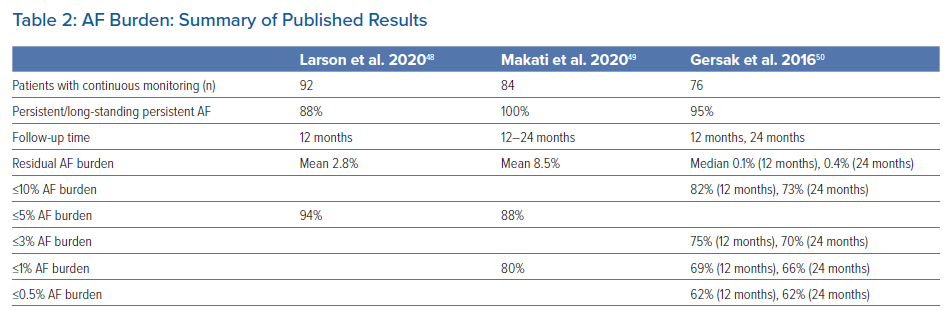
Other studies have also reported on the potential benefit of hybrid epicardial-endocardial ablation on AF burden (Table 2). We evaluated a subgroup of 93 patients at our institution who had continuous monitoring following the convergent procedure.48 These patients had a mean duration of 5.1 years since AF diagnosis with 88% having persistent or long-standing persistent AF. At one year after the post-blanking period, 94% of these patients were free from arrhythmia burden >5%. For those who did have arrhythmia recurrence, their mean burden was <5% with only three patients having higher residual burden. Makati et al. reported residual AF burden in patients with continuous monitoring after the hybrid convergent procedure using cryoballoon for endocardial ablation.49 Of patients with 12–24 months of follow-up post-procedure, 88% had AF burden of ≤5% and mean residual AF burden was 8.5%. Based on implantable loop recorders, Gersak et al. reported 82% and 73% of patients had ≤10% AF burden at 12 and 24 months after the hybrid convergent, respectively.50 Taken together, these studies show that a consistent, substantial proportion of patients have relatively low residual AF burden, which seems to be durable at least 1 year after the hybrid convergent procedure. For patients with advanced AF, consideration of residual arrhythmia burden and change from baseline is important in addition to conventional freedom from arrhythmia recurrence endpoints.
Long-standing Persistent AF
As mentioned above, CONVERGE included 42% long-standing persistent AF patients. This population of AF patients has historically been difficult to treat, evidenced by poor long-term results with endocardial catheter ablation.51 In addition to CONVERGE, other studies have focused on the long-standing persistent AF subgroup. Makati et al. demonstrated favourable outcomes of 70% freedom from atrial arrhythmias at a mean 16.8 months follow-up in a subgroup of 109 patients with long-standing persistent AF.49 Residual AF burden was 7.5% in 57 patients with long-standing persistent AF between 12 and 24 months of follow-up (mean 19.5 months). In 43 patients with long-standing persistent AF, Maclean et al. reported 57.1% of patients with long-standing persistent AF were free from all arrhythmias at a mean 30.5 months from the hybrid convergent procedure, taking into account redo procedures.44
Future Perspective
Another well-recognised site of non-PV triggers that may be important in persistent forms of AF is the LAA.52,53 The BELIEF trial showed an improvement in recurrence-free survival in patients with long-standing persistent AF who received empirical LAA electrical isolation in addition to extensive endocardial ablation compared to those who received extensive endocardial ablation alone.52 This suggests that electrical isolation of the LAA may have a beneficial effect on rhythm outcomes. One caveat of catheter-based electrical isolation is the potential to create LAA contractile dysfunction, which raises concern of potential thrombus formation in the LAA and potential need for continued anticoagulation.52 Epicardial LAA exclusion with a clip device offers electrical isolation as well as mechanical exclusion of the LAA.54 To this end, some investigators have reported the technical approach and outcomes of patients who received both convergent procedure and LAA clip exclusion.37,55–57 Future comparison studies are warranted to evaluate the potential benefit of LAA exclusion in conjunction with hybrid convergent ablation.
While reducing or eliminating atrial arrhythmia recurrence are often the primary endpoints of AF clinical trials, quality-of-life is an endpoint that should not be overlooked or undervalued, particularly in advanced AF treatment. There are several quality-of-life assessment tools, some of which are general, such as the Short Form 36 health survey and others that are specific to AF, such as the Atrial Fibrillation Effect on Quality of Life questionnaire and the Atrial Fibrillation Severity Scale.58–60 In a single centre retrospective analysis, Tonks et al. reported the majority of patients at their centre who underwent the convergent procedure reported reduced AF episode severity and frequency after the procedure, which resulted in significant improvements in quality of life.56 The CONVERGE clinical trial included quality-of-life assessments at baseline and follow-up as secondary endpoints and these outcomes will be a focus of future analyses.41
Conclusion
Cumulative evidence on hybrid convergent procedures, including retrospective analyses and the CONVERGE randomised clinical trial, have shown that the hybrid convergent procedure achieves favourable clinical outcomes in patients with persistent and long-standing persistent AF. These clinical outcomes are improved when compared to similar patients treated with endocardial ablation alone. The impact of hybrid convergent ablation on quality-of-life and additive benefit of LAA exclusion have been preliminarily assessed and are of interest for future studies.
Clinical Perspective
- There is a treatment gap for patients with advanced AF, particularly long-standing persistent AF.
- The left atrial posterior wall is a potential target for ablation in drug-refractory persistent and long-standing persistent AF because it is a site of AF triggers and substrate as AF progresses.
- The hybrid convergent procedure was developed as a minimally invasive approach to effectively isolate the posterior wall and pulmonary veins using combined epicardial and endocardial ablation.
- The prospective, randomised clinical trial, CONVERGE, demonstrated superior effectiveness of hybrid convergent ablation to endocardial ablation in persistent and long-standing persistent AF, and favourable clinical outcomes with hybrid convergent procedures have also been reported in retrospective studies.
- The success of this hybrid approach hinges on the collaboration between electrophysiology and cardiothoracic surgery.