Stroke is a leading cause of death and permanent disability worldwide.1 Ischaemic stroke is the most common stroke variety, comprising more than 80% of strokes in the US.2 One mechanism of ischaemic stroke is atherosclerosis in the extracranial and intracranial arteries, with plaque rupture leading to thrombosis. The second major category is embolic stroke, in which thrombi form in the heart or the arterial/venous beds and then embolise to occlude downstream arteries, typically in the intracranial domain.3 Most embolic strokes are associated with AF. Understanding stroke mechanisms and developing effective risk stratification strategies are crucial for primary and secondary prevention. Control of modifiable risk factors can prevent approximately half of all strokes in high-risk individuals.4
Machine learning (ML) is being increasingly implemented in various disciplines and is emerging as a powerful tool in healthcare. ML offers algorithms capable of modelling complex and hidden relationships between multiple clinical and physiological variables and desired outcomes. A common application of ML in healthcare is precision medicine, where various options are available to treat a particular condition, and tools are developed to predict the treatment protocol most likely to succeed based on the patient’s characteristics.5 Another valuable implementation of ML in healthcare is risk stratification. Prediction of which patients are at risk for a particular disease or associated outcome(s), especially those with greater mortality and morbidity, would enable the use of early interventions and reduce strain on the healthcare system. Most cardiovascular disease (CVD) risk assessment models currently in widespread use are based on conventional statistical methods that incorporate small numbers of predictors quantified by human observers (e.g. left ventricular ejection fraction on transthoracic echocardiography). Such models thus oversimplify complex relationships, including large numbers of risk factors. The emerging field of ML in healthcare, using computer algorithms that learn from a dataset without explicit programming, has the potential to address these limitations and outperform current clinical prediction models. ML models can objectively exploit full datasets (e.g. automated analysis of whole echocardiography clips) and, with the help of multiscale computational modelling, offer patient-specific insights. The ML method used varies depending on the type of data. Classification and regression models (e.g. support vector machine [SVM], random forest [RF], gradient-boosted tree [GBT]) are most commonly used in clinical research. These methods search among the available predictor variables to find the features best linked to the outcome. Deep learning models based on neural networks, especially convolutional neural networks (CNNs), can be used to extract features from ECGs and can be used in image recognition algorithms to find data patterns in image pixels.6
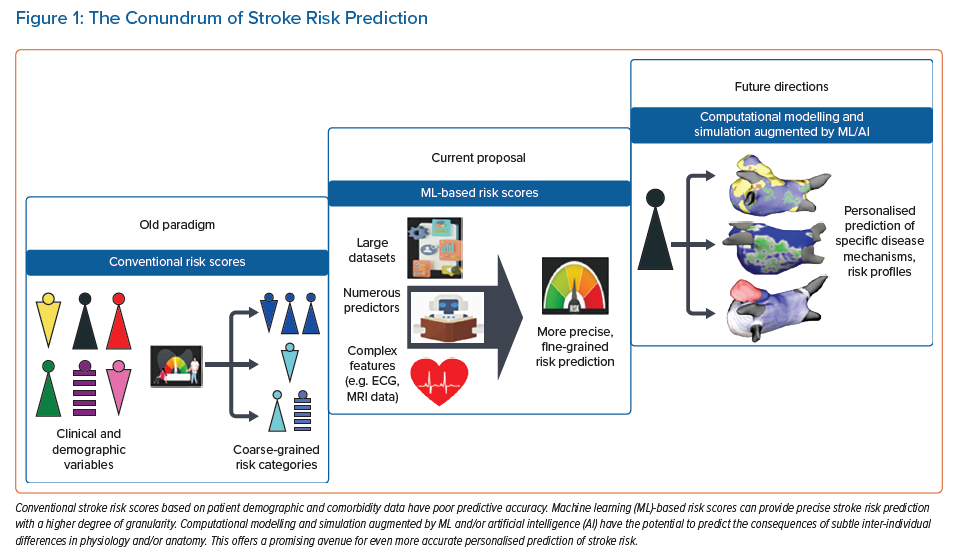
This review discusses risk stratification tools in stroke prediction, exploring new avenues for incorporating ML-based risk assessment (Figure 1).
Cardiovascular Disease Prediction
The term CVD refers to a wide variety of commonly linked pathologies, including coronary artery disease, stroke, peripheral artery disease, deep vein thrombosis and pulmonary embolism. CVD prediction is currently based on risk scores that use patients’ clinical characteristics and comorbidities to approximate the likelihood of future CVD, thus facilitating clinical decision-making. Models widely used in the US include the Framingham Risk Score (FRS) and the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.7,8 Based on the risk profile calculated by these algorithms, the physician may choose to start a patient on statin therapy, for example, in addition to recommending adequate lifestyle modifications.
Machine Learning Models Compared with Traditional Risk Scores
The 2013 ACC/AHA Pooled Cohort Equations Risk Calculator is based on traditional risk factors to predict cardiovascular events over 10 years. It can overestimate or underestimate the overall risk of cardiovascular events in certain groups.9 Kakadiaris et al. used the same risk variables collected in the Multi-Ethnic Study of Atherosclerosis cohort to train an SVM ML algorithm.10 The resulting predictive tool outperformed the ACC/AHA calculator, recommending less drug therapy and missing fewer cardiovascular events. The 2017 Framingham Stroke Risk Profile was compared with ML techniques for stroke risk prediction (random survival forest [RSF], SVM, GBT and multilayer perceptron) in a Chinese cohort of 503,842 adults.11 These ML models improved risk prediction over the conventional Cox model-based approach, with GBT providing the best discrimination and calibration performance. However, the best means of identifying individuals at high risk of stroke was the use of an ensemble model combining the GBT and Cox methods (accuracy of 76% in men and 80% in women). Weng et al. compared four ML algorithms with an established evaluation rubric (ACC guidelines) to predict the first cardiovascular event over 10 years in a prospective cohort of 378,256 patients from UK family practices free from CVD at the study’s outset.12 All ML-based approaches tested were better at identifying individuals at risk of CVD than the established algorithm, with neural networks providing the best risk prediction (area under the receiver operating characteristic curve [AUC] 0.764 versus 0.728 for neural networks versus the conventional method, respectively).
Hung et al. compared deep neural networks (DNNs) with other ML techniques in predicting the 5-year stroke occurrence for a large population-based electronic medical claims database of around 800,000 patients.13 DNNs and GBTs performed similarly high in predicting stroke risk compared with logistic regression and SVM; however, DNNs performed better using fewer patient data when compared with GBT. Four analysis methods were processed and compared in a Chinese hypertension population to assess stroke risk: logistic regression, stepwise logistic regression, extreme gradient boosting (XGBoost) and RF. The best model performance was observed in a random under-sampling applied RF model.14 Wu and Fang found that synthetic minority over-sampling applied to regularised logistic regression outperformed other tested models in an older Chinese population for predicting the risk of stroke.15
Machine Learning Models Can Analyse More Complex Features
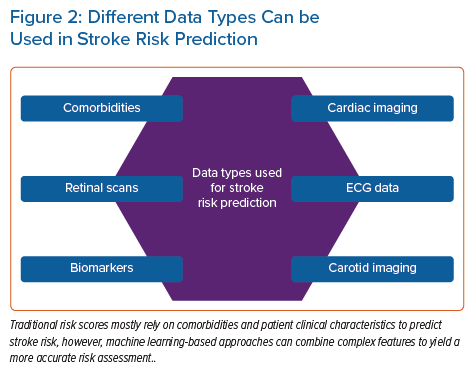
In addition to patient characteristics and demographics, ML can use blood biomarker levels or imaging data as a basis for predicting CVD. Ambale-Venkatesh et al. found that imaging, ECG and serum biomarkers were among the top predictors of CVD as opposed to traditional cardiovascular risk factors and identified RSF as the most predictive cardiovascular risk (including stroke) model out of nine tested models in an initially asymptomatic multiracial population.16 In another study, an ML-based, 27-protein prognostic cardiovascular model was trained and validated to predict the 4-year likelihood of MI, stroke, heart failure or death. The proteomic model performed better than the clinical model in predicting CVD (AUC 0.73 versus 0.64).17 Preliminary results from an ML-based model for predicting white matter lesions as a surrogate for CVD from retinal microvasculature changes (arteriovenous nicking, opacity, focal arteriolar narrowing) are also compelling.18 Four ML classifiers were tested (RF, artificial neural network [ANN], SVM and linear regression), and both the RF and ANN achieved optimal classification accuracy. Thus, retinal imaging is proposed as a screening tool at the primary care level. Sridhar et al. developed a rapid and inexpensive triage tool based on a DNN to predict major adverse cardiovascular outcomes from ECG data in patients with COVID-19.19 This approach yielded comparable results to traditional statistical models incorporating extensive clinical data with the advantage of not requiring clinical expertise to gather medical history. Figure 2 shows the different data types that can be used in stroke risk prediction.
Using a US cohort of 3,435,224 patients from prospective medical databases, Lip et al. showed that the ML approach accounting for the complex and dynamic relationships between variables (including age and incident comorbidities) provided the highest discriminant validity values for stroke prediction (concordance index=0.866).20 This approach may offer a better alternative to the static or single time point evaluations by the traditional risk scores. Although ML models seem to outperform conventional CVD risk scores, and although the existing approach to CVD risk assessment may benefit from an overhaul, additional studies are needed to validate these ML-based models in different cohorts, independent from the ones used in their inception. For example, the ACC/AHA Risk Calculator, one of the most common traditional risk scores, was derived from independent centres and diverse populations, and has been validated multiple times.8
Cardioembolic Stroke
The current clinical paradigm of stroke associated with AF is that arrhythmia, in the presence of cardiovascular risk factors, leads to blood stasis and hypercoagulability (components of Virchow’s triad) with subsequent thrombus formation and systemic embolisation.21 AF is associated with a fivefold increase in stroke risk, and stroke prevention is a cornerstone of AF management.22 Fibrotic remodelling in the atrium plays a central role in the pathogenesis of the arrhythmic substrate for AF.23 It is also increasingly recognised as a significant factor in left atrium appendage (LAA) thrombosis and stroke in AF. Histologically quantified LAA fibrosis burden is higher in patients with AF than in those in sinus rhythm, and in patients with LAA thrombus than in those without.24,25 The mechanism by which atrial fibrosis contributes to thrombogenesis is not completely understood. A decrease in left atrial strain was shown in the presence of extensive late gadolinium enhancement MRI (LGE-MRI) fibrosis.26 Patients with AF had marked alterations in the blood fluid dynamics parameters, particularly a higher mean blood residence time and a lower mean kinetic energy compared with patients without AF.27–30 Whether fibrosis is at the centre of the interaction between overall atrial chamber and LAA remodelling, blood fluid dynamics, AF substrate and thrombogenesis is the subject of ongoing studies.
Clinical Stroke Risk Assessment in Atrial Fibrillation
Prior work aiming to characterise ischaemic stroke risk in AF patients has focused on clinical scores, such as CHADS2, CHA2DS2-VASc and ATRIA. CHADS2 was limited by its difficulty in accurately evaluating low-risk groups.31 To improve the predictive performance in this subset of patients, the CHA2DS2-VASc score was implemented. The ATRIA score outperforms CHA2DS2-VASc in some cases, but both scores remain imperfect predictors of stroke with modest predictive accuracy.32,33
Early efforts to develop ML algorithms for predicting stroke risk in AF patients have shown some promise, and have achieved an AUC as high as 0.892 in one cohort analysis.34 Whereas CHADS2 and CHA2DS2-VASc use 6–7 features to stratify stroke risk, an attention-based DNN model identified up to 48 features that influenced stroke risk using the Korean National Health Insurance data, and provided better prediction of ischaemic stroke in AF patients compared with CHA2DS2-VASc scores (AUC 0.727 versus 0.651, respectively).35 ML was used in a German cohort to predict ischaemic strokes in AF patients started on non-vitamin K oral antagonists. The Sub-population Optimisation and Modelling Solutions (SOMS) tool identified age, male sex, ischaemic heart disease, urinary tract infection, and dementia as predictors of ischaemic stroke in this population.36
Arrhythmia Burden and Stroke
Arrhythmia burden is a significant factor in predicting stroke risk, given that patients with persistent or permanent AF have a higher stroke propensity than patients with paroxysmal AF.37 Catheter ablation to suppress AF has been linked to a reduction in stroke risk in observational studies and in a recent randomised trial of persistent and long-standing AF.38,39 Early rhythm control of patients within 1 year of AF diagnosis has also been associated with stroke reduction.40 Machine-learned signatures of AF burden from continuous remote monitoring data of cardiac implantable electronic devices were superior to CHA2DS2-VASc in predicting stroke, and ensemble models such as RF incorporating CHA2DS2-VASc may be useful extensions to CHA2DS2-VASc alone.41
Anticoagulation reduces stroke risk in patients with AF and risk factors for thromboembolism. Unfortunately, AF is often unrecognised and untreated because it is frequently asymptomatic. A deep learning model can identify patients at high risk of new-onset AF from resting 12-lead ECGs. In patients with no history of AF who develop an AF-related stroke, nearly two-thirds would have been identified pre-stroke as having a high risk for AF via deep learning.42 Distinguishing cardioembolic from non-cardioembolic stroke also has important clinical implications because anticoagulation is generally indicated for patients with cardioembolic stroke. ML-based identification of this subtype of stroke has been demonstrated not only to be feasible using electronic health records, but also helpful in identifying patients who would benefit from oral anticoagulation.43 Wearable devices are currently used to detect AF. In a recent study, a novel photoplethysmography software algorithm had a positive predictive value of 98.2% for concurrent AF. However, detection of AF during periods of active motion remains a challenge, and wearing the devices at night may maximise the sensitivity.44 ML-based models may facilitate accurate real-time prediction of AF onset. Guo et al. developed a photoplethysmography-based ML model capable of predicting AF onset in advance.45 Digital and mobile health technologies can also increase the general awareness of stroke risk factors and prevention.46 The use of real-time detection of AF onset by wearable devices and the guiding of anticoagulation in patients with AF to reduce stroke risk is currently being investigated. Table 1 summarises results from various studies comparing ML algorithms with conventional methods in predicting CVD or stroke.
Embolic Stroke of Undetermined Source
Even after an extensive diagnostic workup, a large proportion of ischaemic strokes are still classified as cryptogenic, including embolic stroke of undetermined source (ESUS). This leaves patients without a treatment tailored to the specific pathophysiology.47 Aetiologies of ischaemic stroke in patients with ESUS include cardiac sources (undetected AF, atrial cardiomyopathy), non-stenotic large artery atherosclerosis, paradoxical emboli related to a patent foramen ovale, and hypercoagulable states. Cardiac LGE-MRI studies have shown comparable levels of atrial fibrosis in ESUS and AF patients.48 ESUS patients with high atrial fibrosis have a high risk of new-onset AF or recurrent stroke.49 Recent computational modelling studies showed that the proarrhythmic substrate properties of fibrosis are similar in ESUS and AF patients, prompting the hypothesis that ESUS patients with fibrotic atria are spared from arrhythmia due to a lack of triggers.50 The subgroup of patients with a cardioembolic aetiology of ESUS could potentially benefit from oral anticoagulation, but correctly identifying this subgroup of patients is challenging. ML may provide novel insights by applying rules extracted from labelled cases (strokes with known aetiology) to unlabelled cases (ESUS). ML models based on patient demographics, comorbidities, laboratory variables and echocardiography can distinguish cardioembolic from non-cardioembolic strokes with relatively high accuracy (AUC ranging from 0.82 to 0.85) and it is estimated that 40–44% of cases of ESUS are cardioembolic in origin.51,52 Undiagnosed paroxysmal AF is often suspected in patients with ESUS and warrants prolonged ambulatory cardiac rhythm monitoring following the stroke episode. In a recent study, artificial intelligence (AI)-enabled ECG was implemented to estimate the risk of paroxysmal AF in ESUS patients; this tool can potentially be used to guide prolonged rhythm monitoring in these patients, however, AF probability by AI analysis of ECGs was not associated with ESUS.53 The fact that AI-enabled ECGs cannot currently predict ESUS reliably highlights the heterogeneous nature of ESUS, and emphasises that it is often caused by mechanisms other than silent AF.
Finally, ML can help categorise ESUS patients into different aetiological groups. A hierarchical k-means clustering algorithm identified four subgroups in a cohort of 800 ESUS patients based on baseline data: arterial disease, atrial cardiopathy, patent foramen ovale, and left ventricular disease. More than half of the patients were assigned to the arterial disease cluster.54 The hypothesis that incorporation of quantitative measurements from advanced carotid and cardiac, including fibrosis, imaging into ML algorithms, improves the classification of stroke aetiology and potentially leads to even more optimal treatment and prevention strategies is currently being tested.
Large Artery Atherosclerosis
Large artery atherosclerosis is a major cause of stroke. Plaque rupture or ulceration often results in the formation of a thrombus that may embolise and occlude a vessel lumen in the brain, obstructing the blood flow and causing a stroke. One way of predicting stroke is via risk assessment using conventional factors responsible for atherosclerosis growth.55 However, this ignores a potentially fruitful avenue for personalisation: each patient’s atherosclerotic plaque morphology, which can be assessed by imaging.56 Integration of carotid imaging phenotypes with conventional risk factors has shown improved risk stratification compared with either method alone.57 However, such approaches still rely on traditional prediction models and have not yet fully leveraged the potential of ML.
Araki et al. described stroke risk prediction by integrating assessment of the near and far walls of the carotid artery using grayscale morphology of the atherosclerotic plaque and showed that this method is superior to the traditional analysis of the far wall of the carotid artery alone.58 Johri et al. measured plaque characteristics using different imaging modalities, and two ML-based algorithms (RF and RSF) were superior to conventional statistical methods in predicting risk of cardiovascular events and coronary artery disease.59 Plaque rupture in atherosclerotic carotid arteries is correlated with high mechanical stresses. Guvenir et al. extracted multicomponent properties of atherosclerotic carotid arteries using ultrasound, MRI, finite element modelling, and ML-based Bayesian optimisation.60 This framework has great potential to be advanced for patient-specific in vivo applications. Wu et al. established an explainable ML model based on XGBoost to predict the presence of carotid plaques in asymptomatic individuals.61 It identified high-risk patients who could benefit from a carotid ultrasound, creating a framework to assist in large-scale stroke screening. Saba et al. developed a deep learning algorithm that can classify carotid plaque into symptomatic (causing one or more transient ischaemic attacks or strokes referable to the appropriate internal carotid artery distribution) versus asymptomatic based on carotid ultrasound characteristics of the plaque.62
Carotid ultrasound imaging can provide valuable data that can help predict CVD and stroke. A low-cost ML-based integrated model taking into account both conventional risk factors and carotid ultrasound image-based phenotypes (CUSIP), using RF as a classifier, showed an improvement of 18% in predicting cardiovascular/stroke risk compared with an ML model incorporating only conventional risk factors.63 The same team later developed an office-based ML cardiovascular risk stratification tool (AtheroEdge Composite Risk Score 2.0) based on clinical risk factors and CUSIP, which provided better cardiovascular risk estimates than the FRS and the WHO risk score.64 Granularity in expressing CVD risk prediction is crucial for personalised medicine. Multiclass ML algorithms can predict several risk categories for a particular disease. Three kinds of multiclass ML cardiovascular risk assessment calculators (based on SVM, RF and XGBoost) showed superior performance compared with conventional CVD risk calculators (FRS, Systematic Coronary Risk Evaluation Score and Atherosclerotic Cardiovascular Disease calculators), with RF performing best.65 Carotid ultrasound imaging, hypertension, use of medications, and smoking had the highest influence on risk prediction. Finally, this team created the first ML algorithm for multilabel cardiovascular event prediction, using both CUSIP and conventional risk factors.66
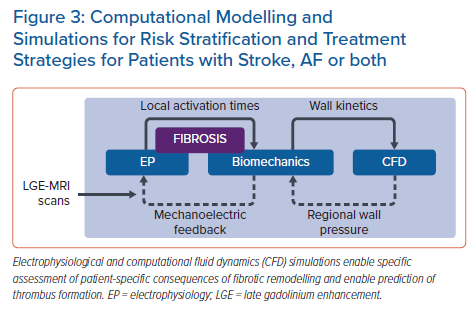
Machine Learning as a Means of Improving or Complementing Computational Simulations of AF
Multiscale, biophysically detailed electrophysiology simulations serve as a complement to conventional experiments and have emerged as a useful approach for deriving insights into arrhythmia mechanisms, with many avenues for patient-specific personalisation.67 Simulations have corroborated AF dynamics observed via intracardiac mapping, which could ultimately lead to improved understanding of AF-related strokes.68,69 ML techniques are being used in this research area, both to improve model reconstruction and as a means of augmenting patient-specific simulation results.
Atrial electrophysiology simulations are typically conducted in models reconstructed from clinical imaging of patient hearts, with regions of fibrosis delineated by LGE-MRI.70 Cell- and tissue-scale bioelectrical properties based on experimental and clinical measurements from human tissue are combined with atlas-based approximations of myocardial fibre orientations in the atrial volume.71,72 Recent developments have exploited ML to improve this process, such as the use of physics-informed neural networks (PINNs) to estimate each individual’s unique fibre orientation by solving an inverse problem based on intracardiac mapping data.73
In parallel research, results from AF simulations are being used alongside other multimodal inputs to ML-based classifiers. Roney et al. reconstructed models from MRI scans in a cohort of 100 AF patients who underwent ablation, then used 10-fold cross-validation to test different ML approaches as a means of predicting long-term recurrence with various inputs.74 Using patient history alone, the optimal classifier (RF) achieved an AUC of 0.61 ± 0.14; when measurements from patient LGE-MRI scans were added, this improved to 0.66 ± 0.17 (K-nearest neighbours with principal component analysis [PCA]). Further augmentation with outcomes from simulations of ablation remarkably boosted the AUC to 0.85 ± 0.09 (SVM with PCA). Working in a separate cohort of 32 paroxysmal AF patients, Shade et al. trained a quadratic discriminant analysis classifier to predict post-ablation recurrence with a similar degree of accuracy (AUC 0.82) from imaging features and simulation outcomes alone.75 Overall, these exciting new findings point towards ML as a powerful means to achieve translationally relevant insights from computational simulations in the context of AF. The hope is that such electrophysiological simulations could ultimately be combined with biomechanics, fluid dynamics and coagulation modelling to better understand each AF patient’s overall stroke risk profile (Figure 3).71
Use of Machine Learning to Better Understand Blood Flow Dynamics and Thrombogenesis
Studies using cardiac MRI and echocardiography show that flow imaging can map intracardiac stasis and predict mural thrombosis and cerebral microembolism.28,29,76–79 However, these studies have been largely limited to the left ventricle and sinus rhythm, given that flow imaging in the fibrillating atrium is challenging. Patient-specific computational fluid dynamics (CFD) analysis has been shown to outperform traditional functional imaging for coronary ischaemia diagnosis and could be a promising tool to assess atrial thrombosis risk.27,30,80–88 However, simulating the flow inside the cardiac chambers requires significant computational power and detailed patient-specific knowledge of inflow/outflow boundary conditions that is often hard to attain (e.g. flow profiles in all pulmonary veins). Although these demands are accessible in research environments, they are a significant barrier to adoption in clinical settings. In the past two decades, there have been significant advances in ML algorithms applied to fluid mechanics.89 ML algorithms, in particular neural networks, have emerged as computationally efficient tools to establish correlations between vascular or chamber anatomy and global flow metrics, including thrombogenic potential.90,91 Neural networks can also be used to declutter or enhance flow imaging data. Two particularly interesting applications are the unwrapping of phase velocity maps and the recovery of wall shear stresses from low-resolution or sparse image data.92–94 Finally, neural networks are widely used in cardiovascular wall segmentation, a crucial step in patient-specific CFD simulations.95,96
Despite their high accuracy and short computation time, many current ML frameworks for flow prediction are trained using large amounts of labelled data from costly, high-resolution CFD simulations, which can be challenging. Training simulations can be performed in patient-specific anatomies, but an alternative approach that saves computational resources is to use simplified geometries and test fully trained ML frameworks on different patients. Thus, this approach is not always fully patient specific. PINNs can alleviate these limitations by prescribing constraints from underlying physical models, including the partial differential equations of fluid motion and their boundary conditions. A paradigmatic example of an application was the prediction of blood flow velocity fields and pressure fields inside patient-specific 3D aneurysm anatomies from contrast agent concentration fields.97 Extensions of this framework are rapidly emerging: two notable examples are the non-invasive measurement of thrombus mechanical properties and of arterial blood pressure from imaging data.98,99 Although the limits of application of these novel ML techniques are still being investigated, it is worth noting that they have the potential to be truly patient specific when provided with sufficient information from a single patient.
Moving Beyond Conventional Methods
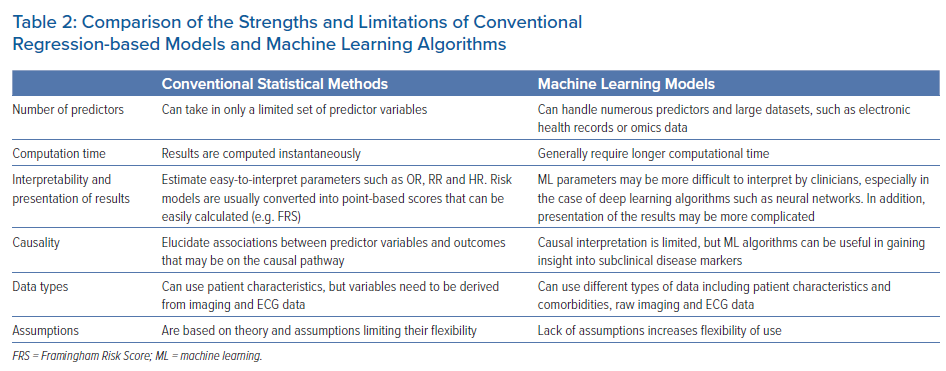
Multiple analytical challenges may not be well handled by regression-based models (Table 2). Regression-based models handle a limited set of risk predictors and fail to provide accurate risk assessment and event prediction when a large number of risk factors is used.6 ML is an alternative approach to traditional statistical inference that offers high predictive power for identifying disease conditions and selecting treatment choices without a deep understanding of underlying mechanisms. Thanks to recent advances in computational hardware and numerical algorithms, ML analysis of large-scale databases of thousands of patients and rich data sources is now feasible. These features make ML a promising new technology to guide prevention and patient management.
Generally, ML methods described in this review outperformed traditional statistical methods in terms of predictive accuracy. The lack of underlying models in ML can be useful in gaining insight into subclinical disease markers that are not captured without assuming causality.16 However, this can also make ML models difficult to interpret and the factors driving model predictions tend to be opaque. For instance, a predictor variable may be selected not because it causes the outcome but simply because it is indirectly associated with a cryptic variable that causes the outcome, which raises practical and ethical concerns.100 The explainability and interpretability of ML algorithms is a subject of active research, and the clinical adoption of increasingly transparent models is highly anticipated.101 Last, ML models differ vastly in computation time: some may be as fast as conventional methods, especially models based on amendments to regression methods and classification trees, while some ensemble approaches can take longer, especially during training and validation.
Although ML seems promising in the stroke prediction realm, there is a need for cautious optimism given that several criteria are essential for the transition of such algorithms into routine clinical practice. Some ML algorithms have been described as a ‘black box’, when there is little insight into how the model is basing its prediction or which variables are contributing most to the predicted risk. In addition, most of these models are based on the impact of a risk factor determined at baseline and outcomes ascertained many years later. Given that stroke risk is strongly determined by aging and incident comorbidities, there is uncertainty in predicting stroke risk in patients with progressive multiple risk factors and comorbidities.
Future Directions
ML models have been increasingly implemented in a variety of healthcare applications and have demonstrated superior predictive value compared with many traditional models for predicting risk of stroke or overall CVD. However, there are no ML-based predictive models approved by the US Food and Drug Administration or similar regulatory bodies for CVD prevention.
For ML to be more widely incorporated in stroke risk prediction, the level of evidence needs to be raised to the same level as that of the studies used to validate current widely used risk calculators. Use of large electronic health records may help achieve this. The use of increasingly large datasets to train, validate and test ML algorithms is expected to lead to better understanding of their theoretical maximum potential. Then, fair comparisons between ML and statistical methods can be fully established and reviewed. Further research is necessary to determine the feasibility of applying ML models in clinical settings and to determine whether this could improve clinical care and patient outcomes.
Clinical Perspective
- The current paradigm of stroke risk assessment and mitigation is centred around clinical risk factors and comorbidities.
- Although functional and easy to use, these models have poor predictive accuracy.
- Machine learning techniques have emerged as a powerful approach to solving data analysis challenges that are poorly addressed by typical regression approaches.
- Multiscale, multiphysics computational modelling, supported by machine learning, holds tremendous promise in providing a mechanistic understanding of thrombogenesis.