Ablative therapies for atrial fibrillation have gained popularity worldwide and prompted the development of new, and often complex, tools to achieve higher levels of success. Pulmonary vein (PV) isolation is widely considered as the cornerstone of successful catheter ablation based on seminal work by Haissaguerre et al.1 Successful isolation of the PVs is now the most commonly sought endpoint in paroxysmal AF, but the optimal strategy in persistent AF remains to be determined. Traditionally, the consensus has been that additional targeting of the atrial substrate responsible for perpetuation of AF is necessary to achieve better outcomes in persistent AF.2 Several strategies have been proposed including additional linear lesions as well as the ablation of complex fractionated electrograms (CFAEs).3,4 This approach has been questioned in light of the recent Substrate and Trigger Ablation for Reduction of AF 2 (STAR-AF 2) study.5 In this multicentre, randomised controlled trial of 589 patients with persistent AF, the addition of either linear ablation or CFAE ablation to PV isolation did not result in improved clinical outcomes. These findings justify a critical assessment of what role, if any, CFAE mapping has in the contemporary era, as CFAEs were traditionally thought to represent areas critical to the perpetuation of AF. This review will provide an overview of the association between CFAEs and underlying AF mechanisms and also the major clinical studies of CFAEs.
Electrophysiological Mechanisms Responsible for Complex Fractionated Electrograms
Definitions
To understand the relevance of CFAE mapping, a clear definition of the term is required. Various mechanisms are believed to contribute to the generation of CFAEs. The resulting complex signals are diverse and CFAE is therefore an umbrella term that describes electrograms with varying characteristics. CFAEs are generally thought to be present when any of the following criteria apply:6–10
- Continuous electrical activity
- At least two deflections
- Cycle length <120 ms
- Amplitude >0.05 mV (to distinguish genuine fractionation from low-level artefact).
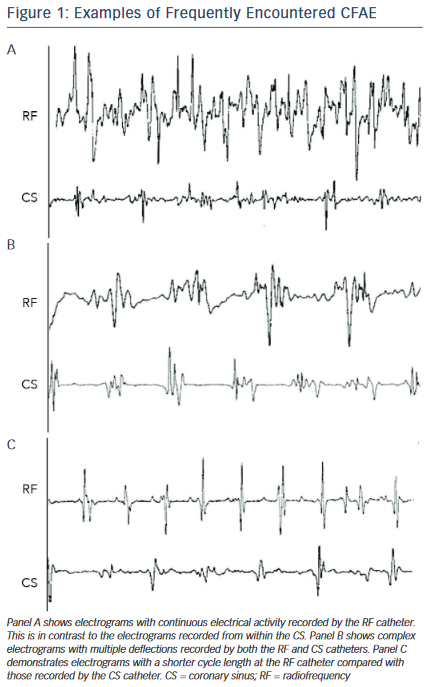
Typical examples of CFAEs are shown in Figure 1. Given this heterogeneity, it is clear that a number of mechanisms are likely to be responsible for the genesis of CFAEs. The use of CFAE mapping as a strategy for AF ablation revolves around the association between CFAEs and AF mechanisms. An important question is whether CFAEs represent local (fixed) properties of the underlying tissue or functional phenomena that vary with time and patterns of activation. At this point it is instructive to refer to the prevailing hypotheses that explain the mechanisms thought to underlie the perpetuation of AF. These mechanisms are not absolutely defined but several hypotheses exist. The focal AF source theory was introduced by Thomas Lewis in 192011 before being superseded by the multiple wavelet hypothesis of Moe and Abildskov in 1959.12 In 1962, Moe hypothesised that a ‘grossly irregular wavefront becomes fractionated as it divides about islets or strands of refractory tissue, and each of the daughter wavelets may now be considered an independent offspring. Fully developed fibrillation would then be a state in which many such randomly wandering wavelets coexist’.13 In this elegant description it is reasonable to make the link between the underlying AF process and the CFAE.
Experimental Support for the Link Between CFAEs and AF Mechanisms
Experimental evidence for the hypothesis linking CFAEs wih AF mechanims was initially derived from animal studies in which excitation of the atria during AF was mapped.14 These studies showed multiple wavelets wandering around natural anatomical obstacles and functional arcs of conduction block. In some cases, the wavelets appeared to be offspring of a single reentrant circuit.14 In the specific example of AF, intraoperative observations have proved instructive in this regard. Konings et al. performed the first systematic mapping of AF in humans by inducing AF in patients undergoing surgery for Wolf-Parkinson White syndrome.15 The free wall of the right atrium was mapped using an electrode containing 244 unipolar electrodes. Regions of continuous electrical activity were most commonly characterised by initial activation by a single wave of leading circle reentry and subsequent collision with other wavefronts. It was inferred that areas of CFAEs during AF represent continuous reentry of fibrillation waves into the same area or overlap of different wavelets entering the same area at different times. Other studies have shown that wavefront collision, functional conduction block, wave break and wave fusion all contribute to the genesis of CFAEs.16,17
The Possible Role of the Autonomic Nervous System
The autonomic nervous system has also been implicated in the genesis of CFAEs. It is recognised that increased vagal tone is associated with easier induction of AF.18,19 Ganglionic plexi are found on the pericardial surface of the heart and provide extensive parasympathetic innervation to the atria. Lemery et al. mapped these plexi by using high-frequency stimulation and assessing for a vagal response.20 One of their main findings was that CFAEs were always reported at sites showing a positive autonomic response. Lin et al. studied a canine model of AF and found that CFAEs could be induced from a hyperactive state within the intrinsic cardiac autonomic nervous system.21 They applied acetylcholine to the atria in varying concentrations during AF and found that the incidence of inducing local CFAE was correlated with the concentration of acetylcholine applied. Injecting acetylcholine into the ganglionic plexi was also shown to induce CFAE. Importantly, CFAE occurring distal from the injected plexi could be attenuated or eliminated by ablation of the plexi thereby suggesting that the occurrence of CFAE did not solely result from a change in local electrophysiological properties, but involved the activation of the neural network within the intrinsic cardiac autonomic nervous system.
The inference from these studies was that some, if not most, fractionated areas were the result of autonomic innervation. This link was further tested by Knecht et al. who used pharmacological blockade (using propranolol and atropine) of the autonomic nervous system to assess the effect on CFAE occurrence and distribution.22 They found that autonomic blockade resulted in only a small reduction in CFAE proportion and this phenomenon was seen only in paroxysmal and not persistent AF. The effect seemed to be mediated by a lengthening of AF cycle as patients with no cycle length change had no alteration in the proportion of electrograms that were fractionated. Interestingly, areas displaying CFAE were stable in two-thirds of cases, again suggesting that the impact of the autonomic nervous system on CFAE is minimal.
Rotors – a Significant Step Forward in Understanding AF Mechanisms?
Kalifa et al. provided new insights into the mechanistic basis of CFAEs during AF.23 They studied a healthy sheep model in which they induced sustained AF. Endocardial optical and electrical mapping of the posterior left atrium was performed to characterise dominant frequencies and the degree of regularity of recorded signals. The use of dominant frequency mapping during AF allows accurate identification of sites of periodic activity. The key findings described were: 1) the posterior left atrium is the site of regular, fast and spatiotemporally organised activity; and 2) highly periodic impulses propagate repetitively from inside to outside the posterior left atrium and fractionate close to the outer limit of the maximum dominant frequency domain, and this outer limit is the region where most fractionated activity surrounds the most regular activity. These apparently stable sources supported the notion of the mother rotor first suggested by Moe in 1962.13 These findings were confirmed by Umapathy et al., who used optical mapping of a murine atrial monolayer model to demonstrate that CFAEs occur at sites of migrating reentrant wavefronts and wavebreak or collision, but not at the core of a stable wavefront.24 The relevance of rotors will be discussed later in this review.
Alternative Sources of Electrogram Complexity and Fractionation
It is important to recognise other potential causes of electrogram fractionation that may not be related to underlying AF processes. CFAEs may reflect purely local effects, but may also be caused by remote activity at the recording site where deflections that result from local and distant activity merge (e.g. the right superior pulmonary veins and the superior vena cava). It is important to recognise the latter point particularly if the identification of fractionated regions is to be used in guiding interventions. Adequate suppression of artefacts by means of efficient signal filtering is also mandatory. Tissue anisotropy may account for electrogram fractionation when activation proceeds in a direction perpendicular to fibre orientation. Finally, misinterpretation of the electrogram may also result from variations in contact between the mapping catheter and the atrial surface that are inevitable when mapping the beating heart. Similarly, electrogram resolution will be affected by differences in electrode characteristics. For example, the resolution afforded by a 4 mm electrode is greater than that provided by an 8 mm electrode. Thus, recognition of tissue and mapping characteristics that might affect electrogram interpretation is crucial when assessing for CFAEs.
CFAE Ablation in Clinical Studies
Given the experimental evidence supporting a link between AF mechanisms and CFAEs, targeting of CFAEs for the purpose of improving AF ablation outcomes has become more prominent. A study by Nademanee et al. suggested that systematic ablation of these regions (as a sole ablation strategy for AF) was associated with excellent acute and chronic outcomes.7 In this study (n=121; 57 paroxysmal and 64 persistent), patients underwent biatrial electroanatomical mapping, and areas associated with CFAEs were identified and ablated with the aim of eliminating CFAE and/or converting to sinus rhythm. Two types of CFAE were described: fractionated electrograms with continuous prolonged activation and electrograms with a short cycle length compared with the rest of the atria. Ablation of CFAE regions (without concomitant PV isolation) resulted in AF termination without external cardioversion in 95 % of patients. One-year follow-up results were encouraging, with 91 % of patients free of arrhythmia or symptoms (the vast majority requiring only one procedure).
Given the crucial role of the PVs as triggers of AF, CFAE ablation without concomitant PV isolation is not widely performed in patients with paroxysmal AF despite the findings of the Nademanee et al. study.7 In the contemporary era, PV isolation alone is widely regarded as the optimal lesion set in paroxysmal AF. Di Biase et al. randomised 103 consecutive patients with paroxysmal AF to one of three ablation strategies: PV isolation alone, PV isolation plus CFAE ablation or CFAE ablation alone.25 At 1-year follow-up after a single procedure, freedom from AF/atrial tachycardia was documented in 89 % of patients in the PV isolation group, 91 % in the PV isolation plus CFAE group and only 23 % in the CFAE group. This point was further emphasised in a meta-analysis of controlled trials comparing PV isolation plus CFAE ablation with PV isolation alone for the maintenance of sinus rhythm.26 Seven trials with a total of 622 participants (with paroxysmal or persistent AF) were included. In patients with persistent AF the addition of CFAE ablation was associated with significantly increased rates of sinus rhythm maintenance (RR 1.35; 95 % CI [1.04–1.75]; p=0.02) at 12 months’ follow-up but this was not the case in paroxysmal AF (RR 1.04; 95 % CI [0.92–1.18]; P=0.53).
CFAE ablation therefore seemed better directed towards the treatment of non-paroxysmal AF, where mechanisms critical for the perpetuation of AF were thought to play a larger role. Whether CFAE ablation alone or with concomitant PV isolation was required remained to be determined. While Nademanee and co-workers demonstrated excellent results in both paroxysmal and persistent AF using a CFAE only ablation strategy,7 other investigators have been unable to replicate these findings. Oral et al. studied 100 patients with persistent AF and performed CFAE ablation within the left atrium and coronary sinus.9 During 14±7 months of follow-up after a single ablation procedure, 33 % of patients had sinus rhythm without receiving antiarrhythmic drugs (versus 77 % in the study by Nademanee et al.). A repeat ablation procedure was performed in 44 % of patients for recurrent AF and/or atrial tachycardia. A striking finding was that PV tachycardias in previously targeted or non-targeted PVs were found in all patients, underlining the importance of PV isolation in ablation of persistent AF. Other investigators have also demonstrated similar findings.4 The marked variation in outcomes between the Nademanee and Oral studies requires some explanation. The Nadamanee et al. study targeted CFAEs in the right atrium as well as left atrium and coronary sinus, whereas Oral et al. did not ablate within the right atrium. Additionally, Nademanee used a 4 mm ablation catheter, whereas Oral et al. used an 8 mm catheter. It is possible that the better resolution afforded by the 4 mm catheter allowed better identification of CFAEs. Furthermore, identification of CFAEs relied on subjective interpretation of the electrogram with obvious limitations.
Automatic CFAE Detection Algortihms
Technical advancement has led to the production of automated algorithms that can be used to identify CFAEs using either the Carto (Biosense Webster) or NavX EnSite (St Jude Medical) electroanatomical mapping systems. One such algorithm embedded in Carto mapping systems identifies low-amplitude, high-frequency electrograms by tagging the peak voltage (positive or negative) exceeding a programmable low threshold (usually ± 0.05 mV) to exclude noise.27 Voltage peaks greater than this threshold, but less than a programmable upper threshold (usually ± 0.15 mV), are identified. The intervals between successive peaks falling within this window are measured. Intervals falling within a programmable duration (usually 60–120 ms) are identified and the number of such intervals during the entire 2.5 s sampling window calculated; this is designated the interval confidence level (ICL). Sites with a greater number of short intervals between low-amplitude multi-deflection complexes (higher ICL) reflect more frequent and repetitive CFAE and thus targets for ablation. Porter et al. found that targeting CFAEs identified using this algorithm resulted in a rate of 1-year freedom from recurrent atrial arrhythmias without anti- arrhythmic therapy of 68 % in patients with persistent AF.28
The algorithm embedded in the NavX EnSite mapping system uses a different principle. It is based on two sequential steps: 1) activation events are recognised in the signal and 2) time intervals between subsequent activations are calculated and their average is denoted as CFAE mean. Locations with cycle length shorter than a pre-specified threshold (nominally 120 ms) are deemed to be ablation targets.29 Application of this more objective approach to CFAE identification was studied in the STAR AF study.4 In this study, 100 patients with high-burden paroxysmal or persistent AF were randomised to PV isolation alone, PV isolation plus adjunctive CFAE ablation (CFAEs identified using the NavX algorithm) or CFAE ablation alone. After one procedure, PV isolation plus CFAE ablation had a significantly higher rate of freedom from AF (74 %) compared with PV isolation (48%) and CFAE ablation (29 %).
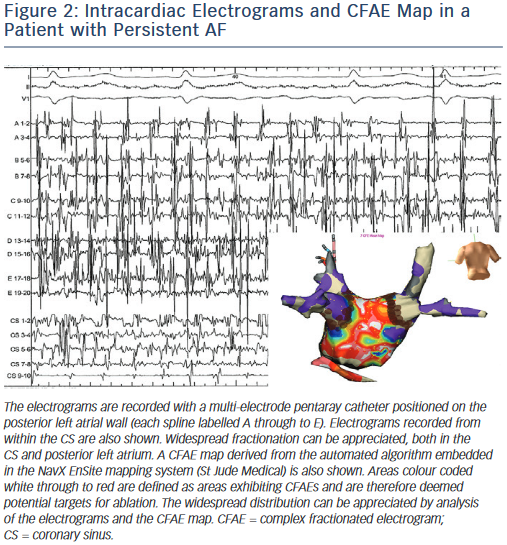
At this stage CFAE ablation seemed to have an adjunctive role in conjunction with PV isolation. Many of the early approaches to CFAE ablation required removal of large (bi)atrial regions; Figure 2 demonstrates a typical example. Several groups have proposed alternative algorithms as methods for reliably identifying CFAEs,30 but in an attempt to reduce the amount of atrial endocardium targeted for ablation, focus has turned towards the concept of a hierarchy of CFAE importance. The notion that some CFAE may represent sites of passive wavefront collision (and therefore not important to the maintenance of AF) is now the subject of much interest.
CFAEs – Active Versus Passive Phenomena
Based on the early experimental data and the subsequent studies demonstrating the effect of distant autonomic effects on the generation of CFAE, the role of CFAEs in the perpetuation of AF has remained uncertain. Yamabe et al. used non-contact mapping to further examine the mechanisms of AF maintenance.17 Focal discharges were found at the pulmonary veins and sites characterised by CFAEs. In this study of 16 patients with paroxysmal AF, focal discharges were identified from the CFAE regions that generated a new wave of activation. Wavebreak and fusion associated with slow conduction and pivoting activation in the CFAE region sustained wave propagation, thereby resulting in the maintenance of AF. These data suggest that CFAEs represent active sources responsible for the perpetuation of AF. It should be noted that this analysis was based on virtual electrograms derived from non-contact mapping which may have made the findings more susceptible to noise and incorrect mathematical extrapolation of the acquired data.
Rostock et al. used a custom-designed pentaray catheter (5 splines with a total of 20 equally distributed electrodes) to perform high- density contact mapping of the left atrium in patients with paroxysmal AF during periods of sustained AF.8 They found that the occurrence of CFAE was significantly associated with a preceding acceleration of AF cycle length. Furthermore, local complex activation/reentry was only seen in 16 % of CFAE regions, with the remaining majority characterised by nearly simultaneous (within 40 ms) activation at all splines. These findings suggest that most CFAE sites are passive and the inverse relationship between CFAE and AF cycle length suggests that distinctive morphological and electrophysiological conditions predispose to the occurrence of fractionation. The same anatomic region may have short, non-fractionated electrograms during an episode with slow AF cycle length, whereas it could have a complex fractionated configuration when AF cycle length is shorter.
The concept that many CFAE sites represent passive phenomena was supported by Jadidi et al. who performed high-density mapping of the left atrium in 18 patients (9 with persistent AF) during sinus rhythm, coronary sinus pacing and AF.31 Only a minority of sites exhibiting CFAE during AF displayed fractionation during sinus rhythm or coronary sinus pacing. Furthermore, there was no correlation between the distribution of fractionation during coronary sinus pacing and sinus rhythm. All sites exhibiting CFAE during AF had normal voltage during sinus rhythm, suggesting the absence of underlying scar. These findings support the notion that the majority of CFAEs are passive in nature and dependent on the direction and rate of activation (coronary sinus pacing versus sinus rhythm versus AF). The fact that most CFAEs do not occur at sites with underlying fibrosis was demonstrated by the same group who performed high-resolution delayed enhancement cardiac MRI to detect atrial fibrosis and compared this with the distribution of CFAE as determined by high-density contact mapping.32 Over 75 % of the regions characterised by dense late enhancement (i.e. fibrosis) did not display CFAE, but demonstrated low-voltage electrical activity.CFAEs were detected at atrial sites displaying voltages >0.5 mV using the EnSite algorithm. However, it is possible that this voltage threshold is too high and therefore may lack the sensitivity and specificity required to identify areas of fibrosis.
With the range of electrograms falling under the umbrella term of CFAE and given that the majority of these appear to be passive phenomena, an important question is whether there is a type of CFAE that is more likely to represent an active participant in the AF process. Takahashi et al. studied 40 patients with persistent AF and performed PV isolation followed by a roof line.10 Electrogram-guided ablation was then performed in the left atrium and coronary sinus.Targeted electrograms were characterised and their association with favourable ablation regions, defined as those associated with slowing of AF cycle length (by ≥6 ms) or termination of AF, was assessed. The examined characteristics of targeted electrograms were: 1) percentage of continuous electrical activity during 4 s; 2) bipolar voltage; 3) dominant frequency; 4) fractionation index (defined as the number of deflections with an absolute value of >0.05 mV from baseline); 5) mean absolute dV/dt of electrograms; 6) local cycle length and 7) presence of a temporal gradient of activation such that there was a >70 ms gradient between activation recorded on the distal and proximal bipoles of the mapping catheter. AF was terminated by electrogram-guided ablation in 73 % of patients. The percentage of continuous electrical activity and the presence of a temporal gradient of activation were independent predictors of favourable ablation regions, and such electrograms may indicate regions that are more likely to be critical to the AF process and therefore good targets for ablation. This hypothesis was tested in the Selective Complex Fractionated Atrial Electrograms Targeting for Atrial Fibrillation Study (SELECT AF). Eighty-six patients with persistent/high- burden paroxysmal AF were randomised to one of two arms: group I, in which all CFAE regions with an ICL >7 (using the Carto algorithm) were ablated, followed by PV isolation; group II, in which only CFAE sites with continuous electrical activity were ablated followed by PV isolation.33 More extensive CFAE ablation (group I) was associated with significantly higher rates of freedom from atrial arrhythmia recurrence at 1-year follow-up (50 % versus 28 %) after one procedure. There were also significantly fewer repeat procedures in this group (13 % versus 36 %, respectively). Adoption of the more generalised CFAE ablation approach targeted a mean left atrial surface area of 22±9 % versus only 3±2 % in the selective approach.
In many cases of persistent AF, areas exhibiting complex fractionation are often widespread. While the SELECT AF study suggested that less selective CFAE ablation was associated with favourable outcomes, numerous mechanistic studies have demonstrated that many CFAEs are only passive participants in the AF process. The dichotomy is possibly explained by the fact that simply targeting continuous activity (as per SELECT AF) is not a specific enough approach for identifying critical CFAEs.
STAR AF 2 – The Last Call for CFAE Mapping?
The concept of more selective CFAE ablation based on electrogram interpretation derived from conventional contact mapping seems to lack supporting evidence. Until recently, the role of CFAE mapping in AF ablation was widely seen as an adjunct to PV isolation in cases of persistent AF. This view has come under considerable scrutiny with the publication of randomised studies calling the benefit of additional CFAE ablation into question. First, the Randomised Ablation Strategies for the Treatment of Persistent Atrial Fibrillation (RASTA) study compared two ablation strategies for persistent AF with PV isolation alone.34 In this single-centre study, patients were randomised to PV isolation alone, PV isolation plus empirical ablation of common non-PV trigger sites, or PV isolation plus CFAE ablation (detected by either the Carto or NavX systems). The primary endpoint of 1-year freedom from atrial arrhythmias without anti-arrhythmic drugs was achieved at a lower rate in the CFAE ablation arm (29 % versus 49 % with PV isolation alone).
A more comprehensive evaluation of the role of CFAE ablation for persistent AF has been provided by the multicentre STAR AF 2 study. In this study, 589 patients were randomised in a 1:4:4 fashion to PV isolation alone, PV isolation plus ablation of CFAEs or PV isolation plus linear ablation across the left atrial roof and mitral valve isthmus. CFAEs were detected using the NavX EnSite Velocity automated algorithm as described above. After 18 months of follow-up there was no significant difference between the three arms in terms of the rate of freedom from recurrent AF (PV isolation alone: 59 %; PV isolation + CFAE: 49 %; PV isolation + linear ablation: 46 %; p=0.15). These findings do not support contemporary guidelines, which suggest that patients with non- paroxysmal AF should have additional substrate ablation to improve outcome.2 The combination of PV isolation plus CFAE ablation plus linear ablation (the so-called ‘stepwise’ approach) was not tested in the STAR AF 2 study, but there are some single-centre data that suggest this may be associated with better outcomes.3 The STAR AF 2 study represents the most robust assessment of ablation strategies for persistent AF and the inference is that PV isolation alone is sufficient.
Are There Better Ways of Assessing the Mechanisms of AF to Identify Critical Regions?
The failure of additional CFAE mapping to improve outcomes in the STAR AF 2 study may in part be due to the CFAE mapping algorithm used.
As discussed above, the threshold voltage of >0.5 mV may be too high for the purpose of detecting atrial regions with underlying fibrosis. Rolf et al. have explored this concept in a pilot study of 178 patients with paroxysmal or persistent AF.35 Patients underwent PV isolation and voltage maps of the left atrium were then constructed in sinus rhythm. Low voltage areas (LVAs; voltage <0.5 mV) were identified in 10 % of patients with paroxysmal AF 35 % of patients with persistent AF. Targeting ablation to these LVAs (after PV isolation) resulted in 12-month atrial tachycardia/AF-free survival of 70 % versus on 27 % in a control group of patients who had LVAs, but did not undergo targeted ablation. Atrial fibrosis is likely to be partly contributory to the mechanisms involved in AF persistence and this method may prove a useful means of targeting such areas.
While the PVs are undoubtedly crucial as trigger sites for AF, understanding of the mechanisms responsible for AF perpetuation remains incomplete and this is in part due to the limitations of conventional mapping strategies. Given that multiple atrial wavelets, macroreentries and localised (focal or reentrant) sources have been reported to contribute to the perpetuation of AF, a fundamental question is whether the many activation waves emanate from a small number of stable, periodic drivers or whether they are transient, widely distributed and self-perpetuating. Conventional mapping techniques lack the temporospatial resolution to determine this and, as already described, CFAE mapping lacks the required specificity. The limitations of conventional contact mapping with a single catheter are emphasised by Atienza et al.36 A high-density dominant frequency left atrium map was created by sequentially moving the ablation and/or circular mapping catheter throughout the entire left atrium. Sites with high-frequency atrial electrograms were identified by an automated algorithm designed to calculate the dominant frequency and depict local atrial activation frequency on the 3D left atrium shell. Targeting these sites for ablation (in addition to PV isolation) did not improve outcomes compared with PV isolation alone. This has led to the development of wide-field mapping tools that incorporate a balloon, multi-spline probes or electrode arrays enveloping the torso.37–40
Narayan et al. hypothesised that AF is sustained by localised sources and that ablation of these sources would improve outcome following AF ablation.39 They developed a novel computational mapping approach to detect these sources and tested whether ablation of these patient-specific sources would modulate AF (termination or significant slowing). They termed this approach focal impulse and rotor modulation (FIRM). In short, a 64-pole basket catheter was used to map the left atrium (and the right atrium in later cases). AF signals were processed by linear detrending, removal of QRS artefact and spatial averaging. Sequences of activation at multiple electrodes in both atria were then used directly to create isochronal maps of AF. Algorithms and software were designed to continually associate electrogram sequences such that rotors or focal beat sources, when identified, could be tracked if they migrated. The electrograms were analysed in the context of rate-dependent repolarisation, that indicate the shortest physiological time between successive activations during AF, and rate- dependent conduction slowing, used to identify mapped propagation paths that were physiologically possible. FIRM maps revealed electrical rotors, defined as sequential clockwise or counter-clockwise activation contours around a centre of rotation, or focal impulses, defined by centrifugal activation contours from an origin. Rotors and focal impulses were only considered AF sources if consistent in multiple recordings to eliminate transient AF patterns.
A total of 92 patients, 76 of whom had non-paroxysmal AF, were studied over 107 procedures.39 Patients were enrolled in a two-arm 1:2 case cohort design and randomised to either ablation guided by FIRM plus conventional ablation or FIRM-blinded ablation (conventional ablation alone). The acute efficacy endpoint was AF termination or ≥10 % reduction in AF cycle length. The primary long-term endpoint was defined as freedom from AF after a single procedure (median of 273-day follow-up). The mean number of AF sources detected per patient was 2.1±1.0, of which 70 % were rotors and 30 % focal impulses. Approximately three-quarters were found in the left atrium, with the remainder in the right atrium. The acute endpoint was achieved in 86 % of the FIRM-guided group versus 20 % in the FIRM- blinded group. Total ablation time was similar between the two groups. The FIRM-guided group had a higher rate of freedom from AF (82 % versus 45 %). These findings therefore offer novel mechanistic insights into the perpetuation of AF and also offer a potentially useful treatment strategy. While these findings need to be confirmed in a randomised, multicentre setting, initial multicentre registry data (78 patients across 10 centres) are encouraging with approximately 80 % of patients being free from AF at 1 year.41
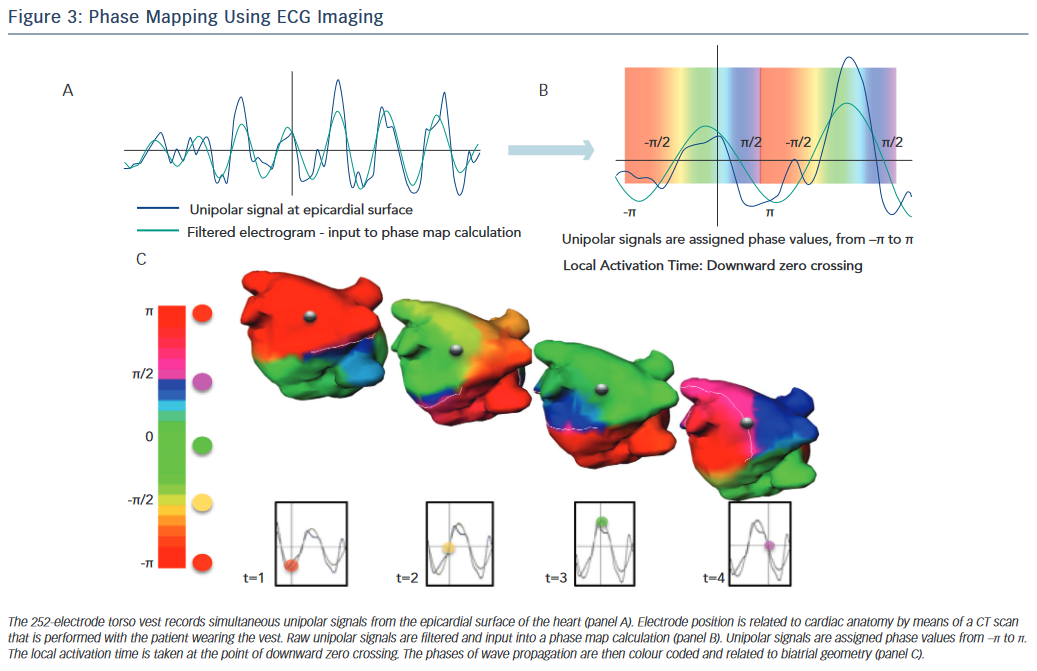
Biatrial AF mapping using activation or phase-based analysis of body surface potentials is another method that has recently been evaluated as a means of visualising the mechanisms responsible for perpetuation of AF.42 This technique takes advantage of the fact that phase parameters are not directly dependent on the amplitude of electrograms, which are often ambiguous during fibrillation. A vest containing 252-electrodes is placed on the torso of the subject and a non-contrast CT scan of the torso performed to derive cardiac geometry and relate this to the position of the electrodes, which record unipolar body surface potentials. This technique has been used to map the origin of cardiac arrhythmias with a localisation accuracy of 6 mm.43
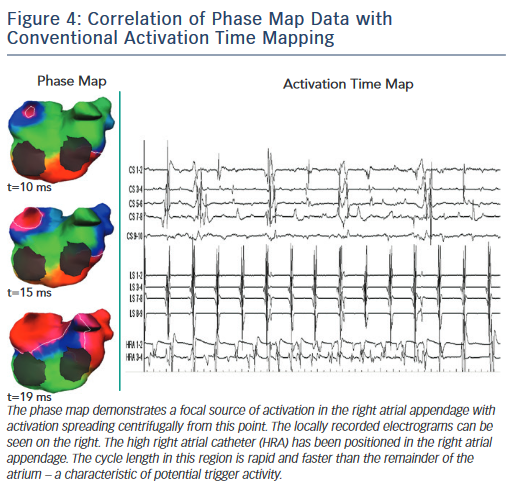
The ECVUE non-invasive mapping system (CardioInsight) reconstructs biatrial unipolar electrograms from the torso potentials. Activation maps can be computed by marking local activation as the steepest –dV/dt of each electrogram. Multiple AF windows lasting >1 s are selected for analysis, and maps of each window are constructed using algorithms that combine signal filtering and phase mapping. A map of each window is displayed on individualised 3D biatrial geometry segmented from the CT scan. The phases of wave propagation are colour-coded. Surrogates of the depolarisation and repolarisation wavefronts are computed from the isophane values equal to π/2 and –π/2, respectively (Figure 3). The maps of each AF window are then combined to produce a composite map of all AF sources that are termed drivers. Figure 4 shows an example of a focal AF driver identified by phase mapping with the associated intracardiac electrograms. Active driver regions are differentiated from passive wave propagation to produce the final spatiotemporal density map.
Haissaguerre et al. used this system to characterise AF mechanisms in patients with non-paroxysmal AF.42 Active driver regions were classified as either focal (centrifugal activation from a single point or area) or reentrant (at least one wave rotated fully around a centre on phase progression and confirmed by sequential activation of raw electrograms). Activity appearing more than once consecutively was considered repetitive. A total of 103 patients with non-paroxysmal AF were mapped. A median of four driver regions was mapped per patient. The number of drivers rose with increasing continuous duration of AF. The ablation strategy in this study was to target driver regions first. If drivers were identified in the PVs, circumferential ablation of ipsilateral PVs was undertaken. If AF still perisisted after all driver ablations had been ablated, linear lesions were undertaken (left atrial roof, mitral isthmus). The primary endpoint of acute AF termination was met in 82/103 patients. Ablation of the driver regions alone terminated AF in 65 patients. Of the 103 patients, 12-month follow-up data were available for 90, of whom 37 were not receiving anti-arrhythmic drugs, and 80% were free from AF (64 % sinus rhythm, 16 % atrial tachycardia). A key finding was the reduction in ablation time when compared with a matched (historical) control group. Ablation time to AF termination was 28±17 mins in the driver ablation group versus 65±33 mins in the control group.
Conclusion
The role of CFAE mapping has reduced in stepwise fashion since its adoption as an ablation strategy for AF. Having initially been proposed as a standalone option in both paroxysmal and persistent AF, it became apparent that its role was perhaps best defined in persistent AF, as an adjunct to PV isolation. This approach has been recently questioned following publication of the STAR AF 2 study. What is clear is that a majority of CFAEs are not active participants in the AF process and as such are poor targets for ablation. The concept of drivers of AF represents a significant step forward in our understanding of the mechanisms responsible for perpetuation of AF and their relationship with electrogram fractionation. Newer wide- field mapping techniques such as ECG imaging and the FIRM-guided approach may represent more optimal means of reliably mapping these active sources. Early data are encouraging but it is important that these are tested in appropriately powered multicentre, randomised controlled trials. Mapping of all CFAEs is almost certainly obsolete, but there undoubtedly remain some CFAEs that are critical sites for AF maintenance and better identification of these may result in better outcomes in persistent AF ablation.