Catheter ablation therapy has been widely used for rhythm control in patients with atrial fibrillation(AF).1 Since the first report in 1994,2 several interventional techniques have been proposed for AF, including replication of the surgical Maze,2 targeting pulmonary vein (PV) foci,3 segmental ostial and circumferential PV isolation,4,5 ganglionated plexi ablation,6 linear lesions in the left atrium (LA),7 complex fractionated atrial electrogram (CFAE)-based ablation8 and a stepwise approach.9 PV isolation remains the cornerstone of paroxysmal AF ablation and has a high success rate. In persistent AF, its role is limited because of the additional involvement of LA substrate due to extensive electrical and anatomical remodelling. The understanding of the substrate maintaining AF leads to the concept of pre-existing specific fibrotic atrial cardiomyopathy. The presence of fibrosis causing changes in cellular coupling results in spatial nonuniform anisotropic impulse propagation, which is a potential cause of abnormal atrial activation underlying the initiation and perpetuation of reentrant arrhythmia-like AF.10,11 In view of these changes underlying persistent AF, several ablation strategies have been attempted to modify the atrial substrate. While there has been some improvement in clinical outcomes,6–9 factors such as undue radiofrequency (RF) application, long procedural duration and high incidence of post-procedural atrial tachycardia (AT) remain.12
Recently, AF mapping using activation13 or phase-based14 analysis of body surface potentials, has allowed potential visualisation of AF-driving substrate in the form of multiple wavelets and reentrant drivers. Prior knowledge of the main AF-driving regions has been shown to facilitate the ablation procedure in patients with persistent AF.14
Non-invasive Body Surface Mapping Analysis for Atrial Fibrillation
From the previous investigations, multiple atrial wavelets, macroreentries and localised sources (focal or reentry) have been reported to lead to maintenance of AF.15,16 Electrical activities in the myocardium during AF can be mapped with electrode arrays in the in situ heart17 and voltage-sensitive fluorescent dyes in the isolated heart,18 which demonstrate complex and often irregular activity. A study involving epicardial mapping of induced human AF demonstrated multiple dynamic wave fronts interacting with changing arcs of conduction block and slow conduction.19 Another recent study mapping chronic AF intraoperatively demonstrated short regular cycle length that could be consistent with a driver with irregular activation of the rest of the atrium.20 Although it is now well accepted that the irregular waveform is seen from an irregular and constantly changing activation sequence on the surface electrogram during AF, these drivers are practically difficult to detect with conventional techniques because of the continuous and intermittent spatiotemporal dynamicity of underlying sources.21,22 To assess driver activities, phase analysis has been applied to these signals. Due to the wavefront interactions leading to constant fractionation and collision, phase mapping provides an amplitudeindependent manner to characterise and visualise dynamic data.23 Non-invasive mapping enables panoramic beat-to-beat mapping of this dynamic rhythm using phase analysis.9,14
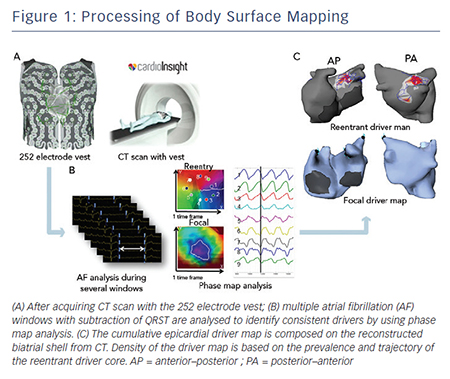
Electrocardiographic imaging, which non-invasively images cardiac electrical activity in the heart, has been developed recently.24–26 This novel modality can demonstrate reconstructed unipolar electrical potentials, electrograms and isochrones on the epicardium by using geometrical information from computed tomography (CT) and an inverse mathematical algorithm.27 Based on the inverse principle, the clinical utility of non-invasive body surface mapping in atrial and ventricular arrhythmias has been reported.28–31 Body surface mapping is undertaken by using a commercially available system (ECVUETM, Cardioinsight Technologies). After acquiring 64 section multi-detector CT images with a vest of 252 body-surface electrodes positioned on the thorax and upper abdomen, epicardial unipolar electrograms are reconstructed on a patient-specific biatrial geometry.27 AF electrograms are acquired during a long ventricular pause – spontaneous or diltiazem-provoked. QRST is subtracted and AF maps are created using specific algorithms combining wavelet transform and phase mapping applied to the reconstructed epicardial potentials. Activation maps are computed using traditional unipolar electrogram intrinsic deflectionbased (dV/dT)max method. The AF drivers can be classified into two categories: (i) focal activation with centrifugal propagation from a point and (ii) reentry/rotor demonstrating rotated wave with full-phase propagation around a functional or anatomical centrepoint. The core and trajectory of reentrant drivers and focal sources are depicted on the patient-specific biatrial geometry (see Figure 1A–C).28,32 The number of foci and reentry through the total duration of all AF windows are displayed on cumulative driver-density maps.
Distribution and Characteristics of Localised Driver
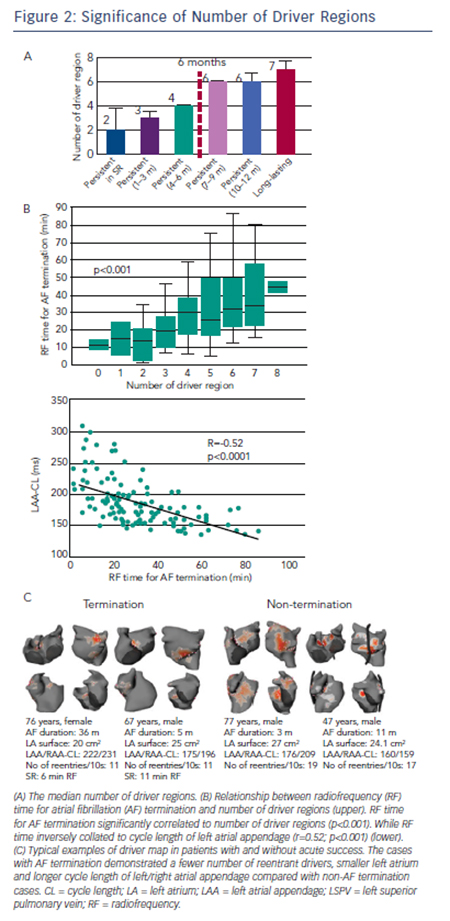
Non-invasive mapping reveals multiple locations of drivers in persistent AF. These drivers demonstrate abrupt appearance at single or multiple sites and reproducibly rotate few times, often showing meandering motion spatiotemporally. In our previous report,14 repetitive reentrant activities (>1 rotation) were observed in 73 % of reentrant drivers with median 2.6 (interquartile range 2.3–3.3) repetitive rotations. The trajectory of reentrant driver core was spread over mean 7±2 cm2; accordingly the map displays driver location not at a discrete site, but over a certain area with variable density (see Figure 1C). The drivers were located in PVs and their respective antra in about 90 % patients in paroxysmal AF where approximately 50 % of patients demonstrated non-PV foci and reentry.33 In persistent AF left PV-appendage (ridge), right PV/septum and inferior LA were most common locations of reentrant driver. Focal driver originated from PV ostium and right or left appendages with a median of four regions.14 The prevalence and distribution of drivers depend on the clinical duration of AF such that the total number of driver regions and activities increase with the length of continuous AF (see Figure 2A), indicating that the driver characteristics are associated with the extent of atrial remodelling. Previous reports demonstrated that longstanding AF had a larger LA surface with a greater amount of total scar (delayed enhancement area on magnetic resonance imaging [MRI]) and more continuous CFAE surface area than persistent AF.34 The relationship between CFAE sites and localised drivers is not clear. In our study, prolonged fractionated electrograms were more frequently ob-served at the reentrant driver regions compared with non-driver regions (62 % versus 40 %; p=0.04). Additionally electrograms recorded on a multielectrode catheter covered most of the AF cycle length at the driver regions, which indicate towards the possibility of localised reentry.14 Of note, multi-electrode endocardial mapping was not performed simultaneously with the body surface mapping. Of note, 50 % of CFAE sites have been reported to be passive bystanders due to non-local signal or wave collisions.35,36 Non-invasive mapping may be considered to reveal critical CFAE sites harbouring localised AF drivers. Interestingly, a previous report showed that 89 % of CFAE sites were located at non-scar and patchy scar areas (41 % at patchy fibrosis area; 48 % at healthy area).34 Another study demonstrated that localised reentrant drivers are located at the border of fibrotic areas,37 with the good correlation between LA fibrosis burden and the number of localised reentrant driver regions (r=0.42; p=0.04). It suggested that the border zone of fibrosis allows formation of a substrate favourable for slow conduction and reentry of wavelets (reentrant driver) capable of perpetuating AF.37
Non-invasive Mapping-guided Ablation
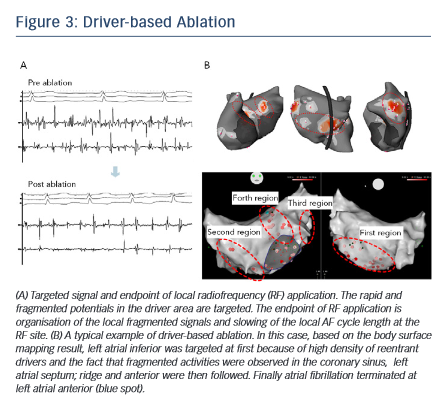
The order of ablation is determined based on the cumulative driver map (see Figure 1C). After acquiring atrial geometry on 3D-electroanatomical mapping system (CARTO3; Biosense Webster Inc), the region having the highest density of reentrant drivers is targeted first followed by the region with the second-highest driver density and so on. Within the driver area, rapid and continuous fragmented signals and the activation gradient between proximal and distal electrodes are locally mapped and targeted for ablation.38 The endpoint of RF application is elimination of fragmented potential and slowing of the AF cycle length at the local site (see Figure 3A). Each RF application targets dominant clusters of AF driver at 30–40 W (25 W in posterior wall) using an irrigated-tip catheter with temperature cut-off set at 45°C. If AF persists after ablation of the first targeted region, the second-highest density area of driver is subsequently ablated in the same way (see Figure 3B). The endpoint of the procedure is AF termination (sinus rhythm [SR] or AT) or completion of RF applications targeting all driver areas. If the drivers are found to be present around PVs, ipsilateral PV isolation is routinely performed. Reentrant drivers are more preferentially targeted than focal drivers because of the fact that sites harbouring atrial focal activities during AF were not found to be strongly associated with AF termination.32 Also, stable drivers with good quality signals on the non-invasive map are targeted regardless of their type. AF sustained after driver-based ablation is electrocardioverted.
Acute and Long-term Clinical Outcome After Driver-guided Ablation
In our series of 103 persistent AF patients who underwent driverbased ablation,14 AF terminated during the procedure in 82 (80 %) patients with RF duration of 35±21 minutes. The mode of termination was SR in 28 (34 %) and AT in 54 (66 %) patients. AF termination with only driver ablation without linear lesions was achieved in 65 (63 %) patients. The number of targeted region for AF termination increased with AF duration (three in AF lasting <3 months, four in AF lasting 4–6 months and six in AF lasting >6 months). Inversely, the termination rate decreased with AF duration (85 % in AF lasting <3 months, 82 % in AF lasting 4–6 months, 67 % in AF lasting 7–9 months, 36 % in AF lasting 10–12 months and 15 % in AF lasting >12 months). Moreover, the patients with SR at the beginning of the procedure demonstrated the highest termination rate of 88 %. An impressive finding of driverbased ablation is that high AF-termination rate was achieved with re-duced RF-time compared with the stepwise approach (28±17 versus 65±33; p<0.0001). At 12 months post ablation, 64 % of patients maintained SR and 22 % were in AT. In this study, 85 % patients with AF termination were free from AF at 1 year after the procedure, which was comparable to stepwise ablation strategy. Also, the AF-free survival rate was higher in patients with acute AF termination than non-termination (85 % versus 63 %; p=0.045). In another population, RF time for AF termination increased with the number of driver regions and correlated to cycle length of left and right atrial appendages (see Figure 2B).39 Continuous AF duration, left appendage cycle length, number of reentrant drives and surface area of LA were independent predictors of AF termination on multivariable analysis (see Figure 2C). These results suggest that driver-based ablation is feasible and effective in patients with persistent AF and those having progressed atrial remodelling with extended fibrosis (AF duration >12 months) continue to pose a challenge.
Recently, a prospective multicentre study (non-invasive mapping of atrial fibrillation study [AFACART]) demonstrated feasibility and utility of body surface mapping in persistent AF.40 In this study, 118 persistent AF patients (AF duration <1 year) were enrolled among eight European centres that had no prior experience using noninvasive system for driver-guided ablation to evaluate its acute success rate, RF time and clinical outcome at 12 months. Acute success (AF termination) was achieved in 64 % patients by driverbased ablation with 46±28 minutes of RF time. During 6±3 months’ follow-up, 83 % of patients were free from AF including recurrent AT in 38 % patients. These results indicate that driver-guided ablation of persistent AF is feasible and its outcomes reproducible in centres with no prior experience.
Limitation and Prospects of Body Surface Mapping
Non-invasive body surface mapping of AF drivers have some limitations: 1) Assessment of the drivers in the septal area is difficult. It is projected posteriorly on right atrium or anteriorly in the interatrial groove from right/left septum. These areas are close to each other and located under the epicardium making it difficult to identify driver area from body surface signals. 2) Agreement between driver areas and ablation sites may not be perfect because the body surface mapping system (ECVUE) does not have 3D-mapping navigation system. Although image integration with CARTO3 is possible, it may not be performed in all cases. 3) Accuracy of detection of small amplitude and very short cycle length (very rapid drivers) signals is not reliable because of the preset limits on the range of acceptable signal/noise ratio and window of analysable AF cycle length (4–8 Hz). The sensitivity is low in the case of far-field and small signals (<0.15 mV), particularly in scar tissue where they may not be detected correctly. In such areas, global activation can be used as a surrogate, yet it may be difficult to distinguish reentrant activity from focal activity. 4) Reconstructed epicardial electrogram signals may be affected by tissues between the epicardium and body surface and specific anatomical conditions (high/low BMI, lung disease, limited coverage of the vest due to body habitus). 5) Transformation of data to phase-based analysis may demonstrate false reentrant activity because of interpolation of incomplete wave curvatures. To resolve this matter, local raw electrograms need to be checked manually to ascertain sequential propagation of regional waves. 6) Finally, reentrant and focal drivers detected by non-invasive body surface mapping were not validated by concurrent endocardial mapping, AF substrates are dynamic 3D structures with a range of discordance between the endocardial and epicardial tissue.41 Hence further examinations will be needed to validate AF mechanisms including reentrant and focal drivers.
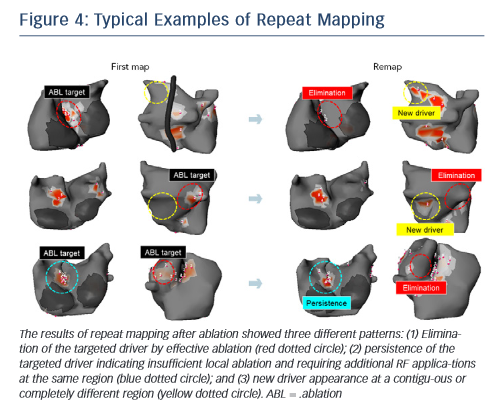
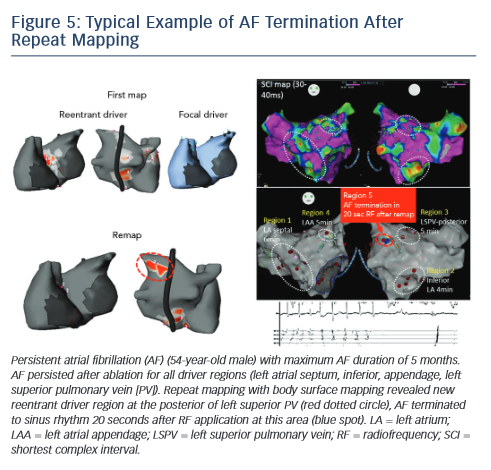
The non-invasive technique has been recently used to remap AF immediately after driver ablation. In fact, the critical point of driverbased ablation is to assess elimination of drivers after ablation. Moreover, incomplete RF application may not impact drivers or generate new AF substrate via slow conduction in the heterogenous region. In a small number of patients, we have shown the results of repeat mapping with different patterns (elimination, persistence and new appearance of driver) (see Figure 4).42Figure 5 demonstrates a typical example of AF termination after repeat body surface mapping. A new reentrant driver appeared at the posterior of left superior PV on remapping AF persisting post driver ablation, AF was terminated by ablation in this area. Repeat non-invasive mapping provides important information on the dynamics of driver domains during ablation. Repeat mapping can reveal residual and new drivers, which have the potential to increase the AF termination rate and improve clinical outcomes.
Conclusion
Non-invasive AF mapping is feasible and guides ablation by providing panoramic beat-to-beat mapping to understand dynamic AF mechanisms. Driver-guided ablation promises equivalent clinical results with less RF time compared with stepwise ablation. Repeat AF mapping post-ablation may provide insights into the impact of ablation on drivers, emergence of residual or possibly new drivers and thereby improve this strategy of AF management.
Clinical Perspective
- Localised reentrant and focal drivers play an important role in perpetuating atrial fibrillation (AF).
- Non-invasive body surface mapping visualises AF drivers in panoramic beat-to-beat mapping and enables understanding of dynamic AF mechanisms.
- Driver-guided ablation achieves similar AF termination rate with a relatively lesser ex-tent of ablation than the stepwise approach, which may improve the clinical outcome in persistent AF.
- When AF persists after driver ablation, repeat mapping allows assessment of the impact of ablation on the drivers and detection of emerging or previously subdued drivers, which continue to maintain AF.