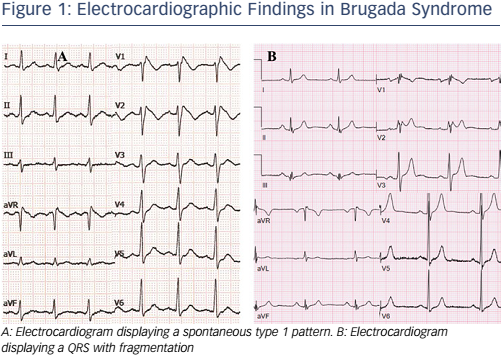
Brugada syndrome (BS) is an inherited disease characterised by coved-type ST-segment elevation in the right precordial leads (V1–V3) and an increased risk of sudden cardiac death (SCD) in the absence of structural heart disease.1
It typically affects otherwise healthy individuals in their forties.2 SCD is the most dramatic presentation, but many patients are asymptomatic at the time of diagnosis.3 As SCD can be the first manifestation of the disease, recognising those patients at risk for future events is of utmost importance. Furthermore, history of warning symptoms might not be present and predisposing factors can be absent prior to the event.4 Asymptomatic patients are at a lower risk of developing SCD, but arrhythmic events are not negligible.2.5
Clinical presentation has evolved since the first description of the syndrome. From the more expressive patients presented in the first reports, nowadays patients are frequently asymptomatic with a nonspontaneous type 1 electrocardiogram (ECG) pattern at diagnosis.6,7
Even after great scientific progress, identifying those patients at risk remains challenging and controversial. Few studies have directly addressed this issue and most available registries are limited to a relative short follow-up period, which makes it impossible to evaluate the whole BS spectrum. As patients remain at risk lifelong, studies with a long follow-up are necessary
Clinical practice guidelines are focused on those patients at higher risk and do not offer specific recommendations concerning the management of individuals who have never suffered an aborted SCD. Specifically, the guidelines only state that an electrophysiological (EP) test might be useful in the management of BS patients with a class IIb recommendation level.8
The placement of an implantable cardioverter-defibrillator (ICD) remains the therapy with the most proven efficacy to prevent SCD in patients with BS. Considering that BS patients have a long life expectancy, device-related complications have to be carefully considered, and the risks and benefits of implantation should be adequately weighed.9
Incidence of Arrhythmic Events
In our experience, 67 % of patients with BS are asymptomatic. Similar figures are found in other registries: 64 % in the France, Italy, Netherlands, Germany (FINGER) registry10 and 79 % in the Programmed Electrical Stimulation Predictive Value (PRELUDE) registry.11 As expected, an asymptomatic status is less frequently found in ICD registries; between 44 and 26 % of patients.2,12
Quantification of arrhythmic events is crucial to offer solid management recommendations. Initial reports showed an event rate of 2.7 % per year in asymptomatic patients.13 This figure has dropped over the years, probably due to selection bias, as initial reports may have included patients at higher risk. Recent registries show an annual incidence of 0.5 % during a mean follow-up of 32–73 months.3,10 In our experience, arrhythmic risk of asymptomatic patients is 3.8 % at 5 years and 4.6 % at 10 and 15 years.3 This might seem relatively low, but when considering the long life expectancy of these patients and the lack of other conditions, this figure becomes very relevant. It should also be noted that the risk of SCD in the general population without BS and at 40 years of age is, at the most, 1:10.000 per year, which is about 100 times less than that for asymptomatic BS patients. Interestingly, when selecting a high-risk asymptomatic population, in whom an ICD has been placed, the rate of events is similar to patients presenting with syncope, underscoring the fact that accurate risk stratification is critical.2
BS has been classically considered a disease that affects mainly men. We have recently reported that BS is not as uncommon in women as previously thought.14 Women represent more than 40 % of our database, with a less severe presentation and more benign course. In asymptomatic women, the event rate is 0.27 % per year, significantly less than men but still significantly more than in the general population without BS. Nevertheless, as will be discussed later, there are no clear risk factors that can help to stratify the risk in women.
Risk Stratification
More than 20 years after the first description of BS, risk stratification remains challenging and controversial. Evidence from big registries and longer follow-ups are now available. Some risk factors have been repeatedly reported and are widely accepted, whilst others remain controversial, with contradictory reports. Furthermore, novel risk markers have emerged and might help when facing an asymptomatic patient with BS.
Current practical guidelines and consensus recommend implantation of an ICD in patients surviving a SCD (class I) and those with syncope and spontaneous type 1 electrocardiogram (ECG) class IIa.8 No specific statement is made for asymptomatic patients. These guidelines do not refer to newly described risk factors and lack recommendations for the low risk, but otherwise frequent, groups.
We will hereafter review the risk factors of importance in asymptomatic patients: both those factors that are widely accepted as well as those that are still controversial or reported less frequently.
Age
Patients with BS are typically diagnosed during their fourth decade.3 Despite age not being related to prognosis, two subgroups merit special consideration: paediatric and elderly patients.
Fortunately, prevalence of BS in the paediatric age group is low. Nevertheless, amongst the eight patients that constituted the initial BS report, three were children. Paediatric BS patients who present symptoms have an especially bad prognosis.4 Conversely, asymptomatic patients appear to have good outcomes, even more so when they do not show the type 1 pattern spontaneously; however, they are not risk free.15 Therefore, individual evaluation is needed and those patients at higher risk should undergo an ICD implantation. Furthermore, the decision to perform a drug challenge (and electrophysiological [EP] study) has to be individualised, balancing risks and benefits.16 However, we believe that it is important to achieve the diagnosis of BS in the paediatric population, to recommend general measures and identify patients at high risk. Importantly, we recommend repetition of the test after puberty, as in our experience, up to 25 % of patients with an initially negative drug test become positive.15
Conversely, BS diagnosed in elderly patients appears to have a benign prognosis.17,18 Furthermore, diagnosis of BS in this age group is infrequent. Amongst all BS patients, those over 70 years of age did not present any arrhythmic events during follow-up17 or were attributed to ischaemic heart disease.18 In this context, decision for implanting or replacing an ICD in elderly patients must be done individually. Available literature is limited to a small number of patients and further evidence is needed. Nevertheless, we believe that establishing the diagnosis is important, as it has family implications. As a familial disease, it is well known that when a patient is diagnosed, a family is diagnosed.
Sex
BS has usually been considered a condition that affects mainly men. We have recently reported that females are not uncommon amongst patients with BS.14 BS in women presents specific differences in comparison with men. Clinical presentation is more benign, with fewer spontaneous type 1 ECG patients and usually presenting as asymptomatic.19 Prognosis is also more favourable, with an annual event rate of 0.25 %. Nevertheless, events can occur during followup,20 and what is even more worrisome is that we lack specific risk factors to stratify this population.14 Few studies have addressed this issue. Benito et al. reported that the history of atrial fibrillation and longer PR interval were associated with arrhythmic events in women with BS.19 We recently found that a previous history of sinus node dysfunction (SND) was related to this prognosis. Interestingly, a spontaneous type 1 ECG is not associated with more frequent events. Furthermore, in our series all the events in asymptomatic women occurred in patients with a drug-induced BS ECG pattern.14
Family History
Previously, the presence of a family history of SCD was not associated with a worse prognosis.3 Our group found that multiple antecedents of SCD in first-degree relatives younger than 35 years of age were associated with further arrhythmic events, but this condition was uncommon and it lost significance when adjusted with other variables.21 When follow-up is expanded over more years, early SCD in first-degree relatives is associated with outcomes of a similar magnitude as spontaneous type 1 ECG pattern (unpublished data).
Genetics
Mutations can be identified in approximately 20–30 % of patients with BS. Some recent reports show a higher proportion.4,22 The presence of an identifiable mutation has not been clearly linked to a worse prognosis; this being particularly true amongst asymptomatic patients.10 Nevertheless, some studies suggest a possible relationship.23 One report showed that certain mutations were associated with the presence of symptoms or longer PR,24 factors known to be related to a worse outcome.
One recent study shows a non-significant borderline association between positive genetic testing and arrhythmic events. Interestingly, none of the negative genotype patients suffered an event. This is an important finding but more evidence is needed as the relationship was non-significant and the population of this study was relatively small.4
Electrocardiogram Pattern
The hallmark of the BS diagnosis is the characteristic ST-segment elevation (see Figure 1). Since the first BS reports, the ECG pattern has shown a clear prognostic value. Patients displaying the spontaneous pattern have a worse prognosis, with a hazard ratio (HR) of 4.0 for events.3 Although patients diagnosed after a drug challenge have a better outcome, they are still at risk.
Furthermore, most asymptomatic patients do not display the spontaneous type 1 pattern. Typical ECG changes might experience spontaneous variations, in both morphology and ST elevation.25,26 In addition to spontaneous fluctuation, many factors and drugs influence ST-segment elevation.27,28 Patients initially considered to have druginduced BS can display the type 1 ECG spontaneous pattern during follow-up. In our experience it can happen in around 20 % of patients.2
A fever-induced type 1 ECG pattern merits a special consideration. Though evidence regarding its value and prognosis is sparse, a recent report shows an arrhythmic incidence rate of 0.9 % per year, an intermediate risk between that of patients with a drug-induced type 1 ECG pattern and those displaying it spontaneously.29
Due to the lower risk of these patients and potential long-term complications of ICD implantation, risk stratification in the drug-induced BS subgroup needs to be precise. Recent reports have questioned the value of drug-induced BS and the drug challenge itself.30,31 To date, drug challenge remains the best available tool for diagnosis. This value was first reported in 2000.32 Unfortunately, the presence or absence of a mutation cannot be considered as the diagnostic gold standard. Furthermore, within the same family, individuals with the same mutation may exhibit different responses during the drug challenge.
Electrocardiogram Parameters
Great effort has been made to find ECG characteristics other than the typical type 1 ECG pattern to identify patients at higher risk. Interesting findings have been reported.
The presence of QRS fragmentation has been associated with a worse prognosis and a more expressive clinical presentation of the BS (see Figure 1).11,33,34 Around one-third of asymptomatic patients might present fragmentation but none suffered arrhythmic events.33 Further reports, however, suggest that it is an independent risk marker and therefore can be useful in asymptomatic patients.34
Together with QRS fragmentation, repolarisation anomalies appear to have value to stratify patients. It has been shown that they can be present in around 10 % of BS patients and might co-exist with fragmented QRS.35 They are associated with a more severe clinical presentation and also have an independent prognosis value. A combination of both parameters might confer patients with an especially high risk.36,37 Interestingly, it has recently been reported that in around 16 % of patients with idiopathic ventricular fibrillation (VF) displaying early repolarisation in the inferior leads, a type 1 Brugada pattern could be recorded in high intercostal leads.38
Other ECG parameters that might be associated with a worse prognosis are T-peak T-end interval, T-wave alternans, the aVR sign and a prominent S-wave in lead I.39--43 Evidence regarding their value is driven by studies involving a small number of patients that were mainly symptomatic. Therefore, their usefulness in asymptomatic patients is yet to be confirmed.
Atrial Fibrillation
Atrial fibrillation is more common in patients with BS than in the general population.44 Furthermore, it can raise clinical suspicion leading to BS diagnosis in a young patient.45 Its presence is related to a higher risk patient, with a more expressive clinical presentation and worse long-term outcome.46 As with other parameters, value in asymptomatic patients is not clear. It can just be a marker of a more severe disease and not independently associated with prognosis. In our experience, it has a borderline association with events that are lost in asymptomatic patients.
Sinus Node Dysfunction
SND can be associated with mutations in the sodium channels.47,48 Not surprisingly, it can be present in BS patients. The underlying mechanism is not clear; a more expressive form of the disease might be involved. SND is usually related to a more severe and early disease, and patients are frequently symptomatic. Nevertheless, we have recently described that in asymptomatic BS patients, concomitant SND has a worse prognosis that might justify a more aggressive therapeutic attitude.3
Electrophysiological Testing
Deep controversy still exists around the prognostic value of an electrophysiological study (EPS). The first data initially suggesting that it might help to identify subjects at higher risk were reported 15 years ago.49 Since then several groups have communicated contradictory results.50,51 In 2010 the FINGER registry was published.10 It pooled data from 11 European centres, 1,029 patients with a median followup of 32 months. Interestingly, they performed a specific analysis of the asymptomatic population. The only variable associated with events was the EPS (performed in 369 patients). When introduced in the multivariable analysis, it lost statistical association (p=0.09), but the number of events in the asymptomatic population was 10 and therefore a lack of statistical power might have been responsible for this result.
Shortly after the FINGER registry the PRELUDE registry was published.11 This study was specifically designed to evaluate the role of EPS in BS. A total of 308 individuals were followed during a median of 34 months. Kaplan-Meier event curves were practically identical in patients with and without induced ventricular arrhythmias (VAs).
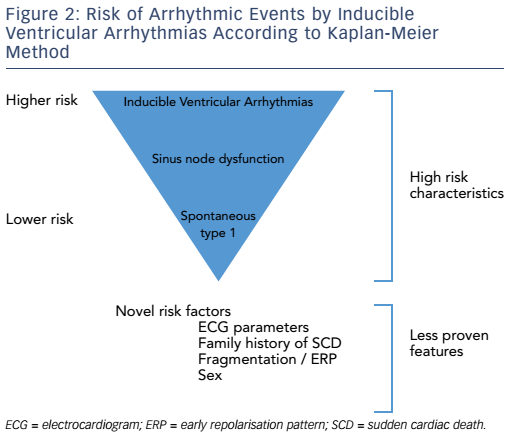
We have recently published our experience in this field.6 Four hundred and four individuals underwent an EPS, after a mean follow-up of 74 months; the EPS was independently associated with a worse outcome (HR 8.3). When restricted to an asymptomatic population it remained predictive for events. Our data suggest that the EPS is useful in both patients presenting type 1 ECG spontaneously or after a drug challenge. Interestingly, the EPS had a high negative predictive value (98.3 %), suggesting that asymptomatic patients with no induced VAs have an excellent prognosis (see Figure 2).
A pooled analysis of the EPS in BS has recently been published.52 It pooled the data from eight registries with 1,312 patients with a median follow-up of 38 months and heterogeneous stimulation protocols. The overall conclusion is that EPS predicts future arrhythmic events with a HR of 2.7. Importantly, inducibility was significantly associated with events when adjusted to a number of variables that included the ECG pattern and symptoms, and was limited to stimulation with up to two extrastimuli. In this study, 53 % of the patients presented a spontaneous type 1 ECG pattern, significantly higher than our cohort. An interesting remark is that this study did not include any data coming from the groups that actively defended the prognostic value of the EPS based on their results. Should those results have been pooled together, the value of the EPS would have been even more significant.
Some considerations should be made around the value of inducible VAs. First, the EPS protocols vary widely throughout the literature. Our protocol has remained unchanged since the first reports. It includes only one stimulation site, the right ventricle apex, and it does not include repetition of extrastimuli. It might be one of the less aggressive in the literature and the fact that our inducibility rate is one of the lowest reflects this. A more aggressive protocol might decrease the specificity of the test and might be the reason for the divergent results found in the literature. The PRELUDE registry used two stimulation sites, and the FINGER and the recently published pooled analysis do not have a homogenous protocol. Furthermore, our population has a more benign profile compared with other studies, with a less spontaneous type 1 ECG pattern; this might explain the difference in the arrhythmic event rate. The second consideration is that we must not forget that the EPS is not a diagnostic test, but rather a tool that helps us to stratify our patients. An inducible VA does not mean that a patient will present arrhythmic events, but only that he (she) might be at a higher risk of sudden death.
General Management Recommendations
An ICD is the most accepted therapy for high-risk patients. Clinical guidelines recommend an ICD in patients that have suffered a SCD (class Ia), patients with syncope and a spontaneous type 1 ECG pattern (class IIa), and those with inducible arrhythmias (class IIb).8,53 Few recommendations are offered for asymptomatic patients.
Risk stratification in BS remains under active investigation. Besides classical risk factors, such as spontaneous type 1 ECG or symptoms, big effort is being made to identify new factors that could help to manage patients. Unfortunately, as previously shown, these new markers are mainly found in patients with a more severe clinical presentation and therefore are easily recognisable as being at high risk.
In our experience, three variables should be taken into special consideration in asymptomatic patients: a spontaneous type 1 ECG, the presence of SND and inducible VAs during programmed stimulation of the heart (see Figure 3).
Inducible VAs demonstrated a HR of 9, the highest amongst the others, and was the only one independently associated with events. Furthermore, recent reports are underscoring its value of the EPS.52
SND should also be considered with special caution. In our experience, a BS patient displaying this condition is at a particularly high risk. Fortunately, it is uncommon, but it is invariably associated with a more severe disease. Most patients with SND are symptomatic and they present at a younger age. When adjusted by other factors, SND loses the statistical relationship with events; however, this might be due to a lack of power.3
ECG findings are also important. Nevertheless, a spontaneous type 1 ECG pattern does not justify on its own an ICD implantation.8 Each patient should be evaluated individually, paying special consideration to other risk factors; such as sex, other ECG characteristics and family history of SCD. In this context a normal EPS is reassuring. A negative predictive value of the test is 98 %, making a patient very unlikely to present future events. In our experience, amongst 289 asymptomatic patients with no inducible VAs, only two presented an event during the EPS.3
ICD implantation should be considered only after a careful evaluation of the risks and benefits. In our experience, around 20 % of patients had inappropriate shocks and 15 % suffered device-related complications.2 These latter complications were found mostly in patients younger than 40 years. Of note, no complication was fatal (though one patient died of a urinary sepsis shortly after a device revision). Similar rates are reported by other groups.9,12 In a study from our group, complications affected up to 33 % of children with ICDs.16 In this particularly active category of patients, lead fracture occurred more frequently. Moreover, the long life expectancy leads to multiple generator change procedures, with a potential increased rate of device-related complications.
Subcutaneous ICDs (S-ICDs) are a promising option in the BS population. Of note, the dynamic ECG pattern that can occur in BS patients might lead to inappropriate shocks.54,55 Furthermore, a small percentage of BS patients might need atrial or ventricular pacing. We have demonstrated that a small percentage of BS patients might have concomitant SND,3 which has prognostic importance and can appear in a paediatric age. Furthermore, monomorphic ventricular tachycardia (VT) might happen in BS and effectively respond to antitachycardia pacing.56 Consequently, S-ICD implantation in BS should be considered after taking into account these facts.
Non-device-based Therapeutic Tools
Quinidine is widely accepted as a treatment for electrical storm or frequent ICD shocks in patients with BS,8 or as an alternative for patients contraindicated for ICD implantation. Quinidine has been shown to be effective as an alternative to ICD, even in high-risk patients. An EP-based drug therapy involves an aggressive electrophysiological stimulation protocol, with repetition of the test under the drug and regularly follow-up. No arrhythmic events have been reported during follow-up in these patients.57 However, other reports showed that quinidine does not completely suppress arrhythmic events in patients with BS,58 and quinidine is not recommended as an alternative to ICD in all high-risk patients. Quinidine acts mainly to inhibit the transient outward potassium current. Given that the mechanisms underlying the development of BS are multifaceted and quinidine’s actions are limited to the inhibition of the transient outward potassium current, drug therapy does not guarantee complete protection.
Epicardial radiofrequency substrate ablation has emerged as a promising tool for the management of BS. First described by Nademanee and colleagues in 2011, radiofrequency ablation of the anterior aspect of the right ventricular outflow tract (RVOT) rendered arrhythmias during electrophysiological testing noninducible, normalised ECG patterns, and had an excellent prognosis at 20 months.59 Similar results have also been reported by others.60 Further experience and evidence is needed as a prophylactic measure in high-risk asymptomatic patients.
Conclusion
Nowadays most patients diagnosed with BS are asymptomatic. Prognosis is more favourable than in symptomatic patients but arrhythmic events happen. Clinical guidelines lack specific recommendations for these patients. Risk stratification remains challenging and sometimes controversial. Spontaneous type 1 ECG, inducible VAs during an EPS and presence of SND can identify patients at a higher risk. Novel risk markers might help in their management.