Cardiac implantable electronic device (CIED) therapy is effective and safe. However, infections related to CIED treatment may have devastating consequences, causing significant morbidity, mortality and generating considerable healthcare costs.1–6 Temporal trends in CIED treatment indicate a disproportional increase in CIED infections relative to implantation rates.1,7,8 Reasons for this trend are uncertain, but likely relate to increasing proportions of implantable cardioverter defibrillator (ICD) and cardiac resynchronisation therapy (CRT) devices implanted, as well as implantations in ’higher risk’ patients, i.e. patients with diabetes, heart failure and renal failure.1
Challenges within the field of CIED infections are multiple with prevention being the most important one. Diagnosis also poses a challenge with many patients exhibiting only vague symptoms.9–12 No international guidelines exist for management of CIED infections; however, an expert consensus statement on lead extractions,13 an American Heart Association scientific statement14 and a British guideline paper15 are useful for guiding decisions. This review aims to present available data along with identification of challenges and outcomes within the field of CIED infection.
Pathogenesis
Bacterial inoculation often occurs as a result of bacterial colonisation of the operative site at time of CIED implantation. Staphylococcus species from the skin, especially, may contaminate the wound, likely during pocket formation, and later cause pocket infection and/or erosion.16 Most investigators concur that the majority of infections seen within the first year are attributed to this early colonisation and the formation of biofilm on device surfaces. Later, pocket erosion may also be caused by operative contamination and biofilm formation. Bacteria in biofilm are protected from killing by host defences and antimicrobial agents.17 Secondary seeding of the CIED may also occur, especially in Staphylococcus aureus bacteraemia. Thus, removal of the entire device is necessary when treating CIED infections.
Incidence of Cardiac Implantable Electronic Device Infection
CIED infection rates vary widely depending on definition and followup duration. In a large, Danish cohort study, the estimated incidence was 1.82/1,000 device-years and higher within the first 12 months in pacemaker (PM) patients.18 Infection risk after PM implant is 0.5–1.0 % within the first 6–12 months.18–20 With more complex CIED types, infection rates are higher; 0.7–1.2 % in ICD recipients21–23 and 1.7–9.5 % in CRT recipients especially with defibrillators (CRT-D).24–26 After CIED replacement and system upgrade procedures, infection rates are 2–4 fold higher than after first implant.18–21,27–30
Presentation and Diagnosis
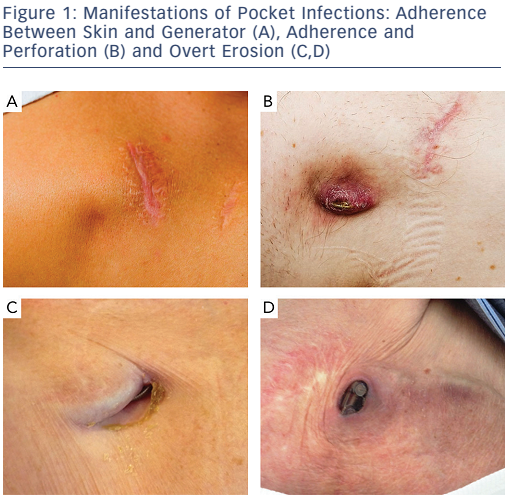
Presentation of CIED infection demonstrates a wide spectrum from subtle complaints of pocket pain to septic shock.9,11,12,19,31 The most common presentation of CIED infection is pocket infection, most often seen within the first year.4,25,32 Pocket infection, however, may present years later, and any symptom from the CIED pocket should raise suspicion of infection and cause patient referral to a CIED specialist for evaluation. Typical signs are local erythema, warmth, pain and swelling, adherence of skin to device, and erosion of skin with a draining sinus (see Figure 1).33 Erosion is de facto infection. Early postoperatively, it may be difficult to distinguish a superficial wound infection from a pocket infection. Percutaneous puncture with pocket fluid aspiration should be avoided in all cases.
Infection may track along the leads and cause bloodstream infection and/or endocarditis. In any CIED patient with systemic infection without obvious focus, CIED infection should be suspected. Blood cultures should be obtained before initiating antibiotic treatment. Cultures should be taken of the pocket and leads when the device is removed. Bacteria typical for CIED infections include staphylococcal species, corynebacteria or propionibacteria, and growth of these supports the diagnosis of CIED infection. Cultures are negative in 15 % of these cases.15 Supplementary diagnostics should include transoesophageal echocardiography to visualise leads, valvular involvement and vegetation,15 and in some cases 18-fluorodeoxyglucose positron emission tomography (FDG-PET).34,35
Prevention of Cardiac Implantable Electronic Device Infection
CIED infection is a serious and potentially fatal complication for the patient, and in general necessitates complete CIED system removal. Thus, prevention of CIED infection is the most important issue.
Before Implantation
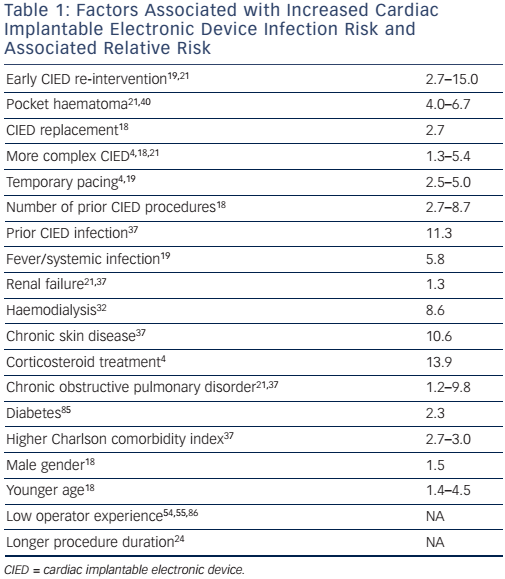
Careful CIED prescription (i.e. evaluation of indication for CIED implantation including appropriate type of CIED) is essential with assessment of risks and benefits, accounting for individual patient characteristics and comorbidities.36 Several patient-related factors are associated with heightened risk of CIED infection, such as age, gender, renal failure, diabetes, respiratory failure, corticosteroid treatment, chronic skin conditions and higher comorbidity index (see Table 1). Since these conditions are largely unavoidable, attention should focus on optimisation before CIED implantation. More complex systems have a higher infection risk and potential benefits from these systems should be weighed against lower infection risk with more simple systems.4,18,21
Meticulous pre-operative preparation is necessary. Fever within 24 hours before implant is associated with a 5–6 fold infection risk, and diagnosis and treatment of ongoing infection before implantation is therefore important.36 It is unsettled whether infected patients who need acute cardiac pacing are best managed with initial temporary transvenous pacing or primary permanent CIED implantation. In most cases, temporary pacing is chosen; however, presence of a temporary pacing lead is also associated with a higher infection risk.19,37
Indwelling lines (e.g. central venous catheters and chest tubes) should be removed before CIED implantation. Most operators prefer >24 hours. Chronic skin conditions are associated with a higher infection risk37 and should be appropriately treated before implant.
Peri-operative
General recommendations for reducing surgical site infections should be applied, including antiseptic skin preparation. Use of chlorhexidinealcohol as an antiseptic reduces surgical site infections compared with povidone-iodine in clean surgery.36,38,39 This effect is thought to be related to a faster and more persistent activity despite exposure to bodily fluids during surgery. Use of transparent films, diathermia or substances to prevent bleeding have not proven beneficial.15
Pre-operative, systemic antibiotic prophylaxis prior to CIED procedures is mandatory. One randomised controlled trial found infection risk reduced to 0.64 % within 6 months with antibiotic prophylaxis versus 3.28 % with placebo.40 Observational data and meta-analyses support this finding.4,18,19,41,42 Supporting evidence for using topical antibiotics is lacking;43 however, recently a promising development has been introduced. The TYRXTM Envelope is an antibacterial envelope releasing minocycline and rifampin in the generator pocket after CIED implantation. This envelope eliminates staphylococcal species and prevents biofilm formation on implanted pacing devices in animal studies,44 and reduces CIED infections in high-risk patients in observational studies.45–47 The most recent version is bio-absorbable and disappears within 9 weeks after implantation. In a single-centre observational study including 1,124 high-risk patients, infection risk was 0.0 % for the bio-absorbable envelope, 0.3 % for the non-absorbable envelope and 3.1 % for controls with minimum follow-up time of 300 days.48 The effect of the bio-absorbable envelope is currently investigated in the Worldwide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP-IT NCT02277990, commenced 2015), aiming to enrol 7,764 patients undergoing a highrisk CIED procedure. Widespread use of this envelope should await the results of this ongoing trial.
Choice of antibiotic prophylaxis differs widely, influenced by local burden of methicillin-resistant staphylococci, local preference and tradition. An ongoing randomised trial enrolling 10,800 patients compares single-dose pre-operative antibiotics (cefazolin or vancomycin) with an antibiotic strategy adding intraoperative wound pocket wash (bacitracin) and post-operative cefalexin/cefadroxil/ clindamycin for two days.49
Prevention of tissue damage with a meticulous surgical technique assuring haemostasis is important. Some authors advocate capsulectomy during CIED replacement procedures to remove avascular tissue. This strategy has, however, not been tested in controlled studies, and capsulectomy may increase haematoma risk, which is associated with a higher infection risk.24,36,40 In anticoagulated patients, continued warfarin use is preferred to heparin bridging because of a lower risk of haematoma (odds ratio [OR] 0.19).50 Use of clopidogrel and aspirin increases risk, however, treatment is rarely discontinued. How to handle patients treated with one of the new oral anticoagulants is less clear. A conservative approach when managing haematoma is often advisable, unless particularly tense or painful. Even large haematomas gradually soften and resorb over a few weeks.
Re-operation
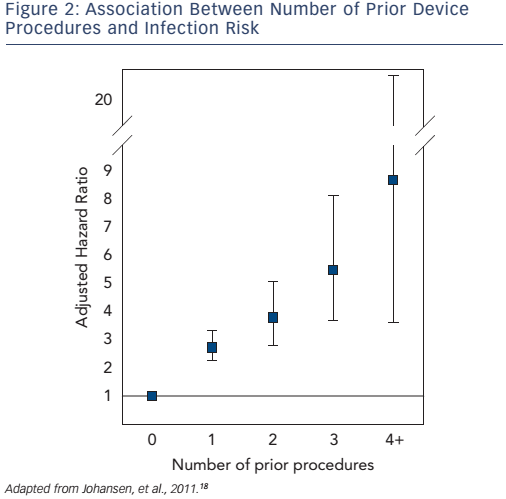
Early re-operation is probably the strongest risk factor for later CIED infection.19,21,24 In a large, Danish cohort study, number of prior CIED procedures was strongly associated with a higher infection risk (see Figure 2).18 Careful CIED prescription is important to avoid early need for system upgrade, and should include estimation of left ventricular function prior to implantation. Attention should be given to reduce anticipated generator replacements with selection of generators with best reported longevity,51 and with careful programming to increase generator longevity. Evaluation of change in CIED indication at time of generator replacement is advisable (e.g. development of permanent atrial fibrillation or chronic heart failure). Need for CIED upgrade should be evaluated extremely carefully, given the high infection risk.21 Use of active fixation leads should be advocated – with due consideration for a suspected minor increased risk of cardiac perforation – to reduce risk of lead dislodgement and need for re-operation.52 Appropriate education and reasonable implant volume for each operator should be assured to decrease risk of lead dislodgements, and complications in general, requiring early re-operations.20,52–57
New Advances
The recently introduced leadless PMs58 are likely to bear a lower risk of infection than transvenous systems; however, in the event of infection, extraction risk is unknown especially years after implantation. Confirmation of long-term efficacy and safety that is at least non-inferior to traditional PMs in randomised controlled trials is needed before advocating for their widespread utilisation. Currently, no such studies are underway.
Subcutaneous ICD systems59 do not bear the risk of blood-stream infection or endocarditis seen with traditional transvenous ICD systems. They could be attractive for patients not needing bradycardia or antitachycardia pacing and at particularly high risk of CIED infection. It is unlikely that the pocket infection rate is much lower than for traditional PMs. Again, documentation of at least non-inferiority compared with transvenous ICD systems is needed before expanding this treatment to larger patient-groups. The extraction risk, though, is negligible.
Treatment of Cardiac Implantable Electronic Device Infection
Confirmation of CIED infection regardless of systemic or localised to pocket mandates prompt removal of all CIED hardware, and a prolonged course of intravenous antibiotics. Furthermore, a strategy for re-implantation is warranted. However, in cases of minor incisional abscesses, a few days after implantation, a course of antibiotics and careful follow-up may be sufficient.15
Planning of Treatment
Planning of timely, correct and complete treatment is of the highest importance for better patient prognosis. Partial procedures such as generator removal and capping of leads are associated with an almost invariable relapse10,33,60,61 regardless of clinical presentation. General consensus favours percutaneous removal in centres with procedural volume sufficient to maintain operator skills, and with immediate surgical backup.13,14,62
Management of CIED infection is a multidisciplinary task, and may involve many specialists and various imaging techniques.63 Strategy for antibiotic treatment is often directed by an infection disease specialist. Few data exist in this field, but generally, antibiotics are recommended for 10–14 days after pocket infection, 14 days for bacteraemia and 4–6 weeks for endocarditis.14 Pacemaker-dependent patients pose a particular challenge, and temporary pacing needs careful planning. Heart failure teams should be involved after CRT device removal when anticipating haemodynamic support. All CIED hardware, including abandoned leads, even if contralateral to infected system, must be removed. In preparation, a chest X-ray is important and computerised tomography (CT)-scans may be used to visualise suspected perforated leads. Transoesophageal or intracardiac64,65 echocardiography is an important part of the evaluation.14 Large vegetations exceeding 2 cm may indicate surgical explants.13,31
Extraction methodology is beyond the scope of this article.
Re-implantation
Plan for re-implantation is advisable before system extraction. CIED indication must be re-evaluated because some arrhythmia problems may have resolved. Observational studies found that 20–40 % of patients were discharged without CIED re-implantation.32,66,67 Some patients have developed indication for more complex CIED treatment while others will need no re-implantation at all (e.g. malignancy in patients with prophylactic ICD). When re-implantation is necessary, this should be done on the opposite side of the chest.
Timing of re-implantation must be individualised, with careful attention to an adequate period of antibiotic therapy. Epicardial placement of PM leads may be considered for those at high risk of re-infection or with limited vascular access. Leadless PM and subcutaneous ICD implantation could also be considered for selected patients. A continued dialogue, beginning pre-operatively, among the electrophysiologist, the surgeon and the infection disease specialist, is critical to ensure individual management plans.
Outcomes of Cardiac Implantable Electronic Device Infection
In general, extraction of all CIED hardware is needed for successful treatment of CIED infections. A series without complete device removal have shown that over half of patients demonstrate relapse.10,68,69 In a recent study, it was found that the rate of CIED infection after CIED extraction was higher in patients with incomplete lead removal, 13.5 % versus 3.0 %.70 In addition, mortality appears higher without complete removal.71–73 One study indicated a threefold higher mortality without system removal.74
All-cause mortality following CIED infection is considerable, ranging from 6 % to 35 % at 2 years or longer follow-up, although many deaths are not infection-related.10,66,70,74–76 In most of these studies, more than 90 % of patients underwent complete CIED removal. Mortality with endocarditis is reported between 24.5 % and 29.0 %, 31,71,77 and CIED infections with endocarditis have a higher mortality than pocket infection.3,78 One study found a 6-month mortality of 10.1 % after system removal in patients with small lead vegetations and 18.4 % in patients with large lead vegetations.31
System extraction is associated with a small risk of major complications, particularly vascular lacerations causing haemothorax and death.32,70,79–82 Extraction-related mortality rates are 0.1–0.8 % in large experienced centres.70,83 In one large, single-centre study, including more than 5,000 lead extractions, 43 % of which were due to infection, risk of major complications was 1.8 %, and minor complications 3.6 %. A total of 11 patients died as a result of the procedure.84 Complications are typically more frequent in surgical extractions with open heart surgery.31
Conclusion
CIED infection is an increasing problem due to rising absolute numbers of CIED procedures and increasing patient comorbidity. The key challenge in the management of CIED infection is prevention. Careful prescription of CIED treatment and careful patient preparation before implantation is important. Diagnosis is often difficult and delayed by subtle signs of infection. Treatment of CIED infection includes complete system removal in centres experienced in CIED extraction and prolonged antibiotic therapy. Meticulous planning and preparation before system extraction and later CIED re-implantation is essential for better patient outcome. Future strategies for reducing CIED infection should be tested in sufficiently powered and welldesigned, multicentre, randomised controlled trials.
Clinical Perspective
- Cardiac implantable device (CIED) infection is an increasing problem.
- The key challenge in management of CIED infection is prevention.
- Careful CIED prescription and preparation before implantation is important.
- Diagnosis of CIED infection often is difficult due to subtle signs of infection.
- Treatment of CIED infection includes complete system removal and often prolonged antibiotic therapy.