Over the last few decades an increasing body of evidence has supported the role of cardiovascular implantable electronic devices (CIEDs) including permanent pacemakers (PPMs), implantable cardioverter-defibrillators (ICDs) and cardiac resynchronisation therapy (CRT-D [with defibrillator] and CRT-P [without defibrillator]) in improving quality of life and survival.1 In addition, there has been a significant increase in the number of implantation procedures and subsequent replacements, revisions and upgrades.2 Between 1993 and 2008, 4.2 million patients underwent implantation of a CIED.3 A worldwide cardiac pacing and ICD survey, which included more than 80 % of all the pacemakers and ICDs implanted worldwide during 2009, reported 737,840 new implants and 264,824 replacements, a significant rise compared with a similar survey conducted in 2005.4
However, the cost and complications of device implantation, including infection or hardware malfunction in patients receiving CIEDs, have led to the concern that negative outcomes may partially counteract the expected benefits. The rate of CIED infection has been estimated at 0.5 % with primary implants and 1–7 % with secondary interventions.3,5–8 It is difficult to give accurate estimates of infection rates, given the fact that figures are partly based on retrospective series of varying duration, and that different definitions of infection exist. However, the incidence of CIED infection is increasing out of proportion to CIED implantation.3,5,9 A US study reported a 12 % increase in the number of CIED implantations from 2004 to 2006, with a 57 % increment in CIED infections during the same period.9 Reasons for this rise in CIED infections include the fact that younger patients are receiving CIEDs, and therefore surviving long enough to require more pulse generator changes and lead revisions, which are associated with a higher infection rate.7,10 In addition, there has been an increase in comorbidities, such as diabetes and kidney disease, resulting in poor wound healing and diminished immune responses.3,9,11,12 Expanding indications for CIED use, coupled with an ageing population with more comorbidities, mean this trend is likely to increase.2 Better awareness and improved reporting of CIED infections may, however, help to decrease the higher complication rates noted in recent years.
CIED infections also impose a substantial financial burden resulting from prolonged hospital stays, long duration of antibiotic therapy, management of sepsis and complications, device extraction and reimplantation.3 These infections typically cost at least $52,00013 and may exceed $100,000.14 This article will review strategies for management and prevention of CIED infections, including lead extraction and the use of an absorbable antibacterial envelope.
Risk Factors for Cardiac Implantable Electronic Device Infections
Risk factors for CIED infections include patient factors such as medical comorbidities,15 renal failure,15–17 heart failure,16,18 diabetes,16,18 fever within 24 hours before the implantation,7 anticoagulation17 and steroid use.19 Important device-related risk factors include device revision or upgrade,8 the use of more than two pacing leads and the need for early pocket re-exploration.7,19 The presence of multiple leads increases the risk of central venous thrombosis in the area of the leads and is a potential site of secondary seeding of bacteria.20 Procedure-related factors include procedure time, temporary pacemaker use prior to implantation, early re-intervention and postoperative haematoma at the device pocket site.21 ICD replacement is associated with a 2.5x greater incidence of pocket-related events, and the need for re-intervention increases with every consecutive replacement.10
A registry study found that PPM and ICD generator replacements were associated with a substantial complication risk, particularly those with lead additions.8 A study of 122 ICD patients undergoing generator replacement or surgical lead revision between January 2006 and July 2008 found that one-third of patients had an asymptomatic bacterial colonisation of the generator pocket. After revision, 7.5 % of these patients developed a device infection over 108 ± 73 days with the same species of microorganism.22
However, these risk factors have mostly been derived from small, single-centre studies. There is a need for larger, more representative studies to identify the most important factors that are responsible for the development of CIED infection. There is no consensus definition of patients at high risk of CIED infection; a composite risk score has been proposed,23 but definitions of high-risk patients vary across studies.
Pathogenesis, Presentation and Diagnosis of Cardiac Implantable Electronic Device Infection
CIED-related infections are mainly due to local contamination during implantation; breach of the skin barrier introduces bacteria into the device pocket.24 The majority (88 %) of CIED infections are caused by Gram-positive organisms;24–26 the most common organism is methicillin-sensitive Staphylococcus aureus (MSSA; 30.8 %), followed by coagulase-negative Staphylococcus (20.5 %). Around half of these Staphylococcus infections are methicillin-resistant Staph. aureus (MRSA).25,27 The majority (60 %) of CIED infections are pocket infections, characterised by erythema, tenderness, warmth and erosion,28 but infection can track along the intravascular portion of the leads, leading to intravascular infection, manifesting as bacteraemia and endocarditis.29–32 A study found that, even when infection symptoms were limited to the device pocket, in 72 % of cases the intravascular segments of the leads had positive blood cultures.31 Less commonly, the intravascular portion of the CIED can become infected as a result of haematogenous seeding from another infection site, and vegetations on the leads are frequently detected by transoesophageal echocardiography (TOE).33 Early presentations typically result from wound infections, MRSA or MSSA. Late presentations are more likely to be related to vascular access.12,34
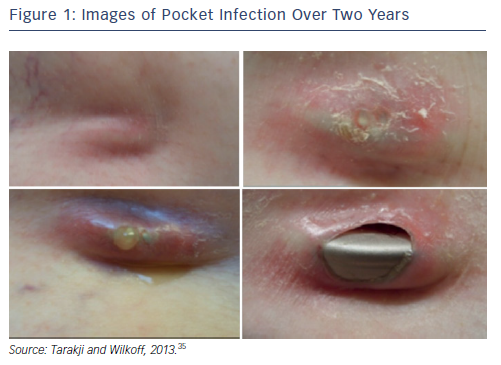
It can be hard to diagnose CIED infections since numerous conditions can present with the same symptoms. Clinical manifestations of CIED range from local device pocket erosion to full-blown sepsis,25 and include symptoms such as erythema, warmth, tenderness, purulent discharge, erosion of generator or protrusion of leads through the skin (see Figure 1).26,35 Furthermore, up to 30 % of patients present with nonspecific symptoms only, such as fever and malaise.
In order to diagnose systemic CIED infection, two sets of blood cultures should be obtained before initiating antibiotic therapy (class I recommendation). Percutaneous aspiration of the pocket should not be performed (class III recommendation). The use of transthoracic echocardiogram (TTE) should be mandatory to investigate the possibility of endocarditis.28 The results should be interpreted according to the individual patient; for example a positive echo density does not always indicate infection and a lack of vegetation in the blood culture does not eliminate the possibility of CIED-related bacteraemia.
Patients with bacteraemia but no evidence of device pocket infection or endocarditis represent a diagnostic challenge. The use of TOE is essential in this group of patients as TTE lacks the necessary specificity.36 The diagnosis may only be confirmed if infection relapses or persists after completion of the antibiotic course, particularly in the case of Gram-positive organisms other than Staph. aureus.28,37 Given the increasing prevalence of CIED infections and the occasionally challenging nature of diagnosis, particularly in the absence of pocket involvement and with negative TOE, other diagnostic techniques have been investigated. Several show promise, including the use of 18F-fluorodeoxyglucose–PET/CT.38,39
Management of Cardiac Implantable Electronic Device Infection
Correct management of patients with CIED infection depends on the clinical presentation and the causative pathogen. In mild cases such as superficial incision site infection or stitch abscess, conservative management strategies may suffice, such as 7–10 days of antimicrobial therapy and removal of the stitches.40 When CIED infection is restricted to the pocket site, an American Heart Association (AHA) scientific statement recommends 7–10 days of therapy after device removal if no inflammatory changes are seen, otherwise 10–14 days of antimicrobial treatment is recommended.28 At least one of the authors would extend the treatment until complete wound healing. Antibiotic treatment in cases of systemic CIED infection is more uniform, usually involving 4–5 weeks of IV treatment, also depending on the type of causative bacteria.
The Heart Rhythm Society (HRS) and European Heart Rhythm Association (EHRA) recommend complete device and lead removal in all patients with definite CIED systemic infection as evidenced by valvular endocarditis, lead endocarditis and sepsis. It is also recommended for all patients with CIED pocket infection as evidenced by pocket abscess, device erosion, skin adherence or chronic draining sinus without clinically evident involvement of the transvenous portion, patients with valvular endocarditis without definite involvement of the leads and/or device and in patients with occult Gram-positive bacteria.41,42 Complete device and lead removal is also reasonable in patients with persistent occult Gram-negative bacteria, but is not indicated for superficial or incisional infection without involvement of the device and/or leads, nor to treat chronic bacteraemia due to a source other than the CIED, when the source could not be eliminated and long-term antibiotic treatment is required.41 Satisfactory control of the infection is required before implantation of a replacement device may be considered.
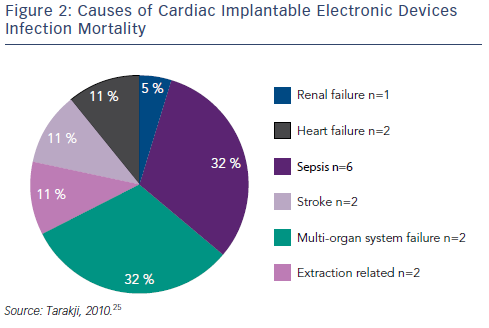
Transvenous device system explantation is the preferred technique; intraprocedural risks include haemothorax, laceration of the superior vena cava, damage to the tricuspid valve and cardiac tamponade.25,26,28 However, without system removal the rate of relapse is high: rates of 50–100 % have been reported, compared with 0–4.2 % for complete system removal.26,27,31,43,44 Mortality can be as high as 31–66 % if the device is not removed.30,45 Using a combined approach with antibiotic and CIED removal, the one-year mortality is still approximately 20 %.30,45,46 Data from the National Hospital Discharge Survey from 1996–2003 demonstrated that CIED infection doubles the rate of in-hospital mortality.47 In one study that included 412 patients with CIED infection, the causes of in-hospital mortality are shown in Figure 2. Of note, only two out of the 19 in-hospital deaths were related to CIED extraction.25 Delays to treatment and incomplete system removal are associated with higher mortality.3,14 Recent data from the European multicentre study on lead extraction (ELECTRA) also show a significant in-hospital mortality of CIED infection patients, however only a minority of deaths were related to the extraction procedure.48 In a study investigating the clinical predictors of shortand long-term mortality in patients with CIED infection, the following risk factors were identified: patient age (hazard ratio [HR] 1.20, 95 % CI [1.06–1.36]), heart failure (HR 2.01, 95 % CI 1.42–2.86), metastatic malignancy (HR 5.99, 95 % CI [1.67–21.53]), corticosteroid therapy (HR 1.97, 95 % CI [1.22–3.18]), renal failure (HR 1.94, 95 % CI [1.37– 2.74]), and CIED-related endocarditis (HR 1.68, 95 % CI [1.17–2.41]).49
The need for transvenous lead extraction (TLE) has been increasing in proportion to the increased number of CIED implantations. In a study of patients undergoing TLE, a total of 5,973 (4,436 [74.3 %] PPM and 1,537 [25.7 %] ICD) leads were extracted during 3,258 TLE procedures.50 Among these, 25 (0.8 %) patients experienced major complications requiring emergent surgical or endovascular intervention. Twenty patients (0.6 %) underwent sternotomy (n=18) or thoracotomy (n=2) for superior vena cava laceration (n=15) and right atrial (n=2) or ventricular (n=3) perforation. Two patients required vascular repair at the access site for subclavian vein or artery laceration. In-hospital mortality was 36 % including six procedural/operative deaths (0.2 %).50 Factors associated with increased procedural complications (not mortality) risk include body mass index (BMI) <25 kg/m2, damaged leads and ICD leads.42 Predictors of major complications associated with TLE include cerebrovascular disease, ejection fraction ≤15 %, lower platelet count, international normalised ratio ≥1.2, mechanical sheaths and powered sheaths.51 Thirty-day all-cause mortality following TLE has been associated with BMI, haemoglobin, end-stage renal disease left ventricular ejection fraction, New York Heart Association (NYHA) functional class, extraction for infection, number of prior lead extractions performed by the operator and extraction of a dual-coil defibrillator lead.52
Procedural success can be enhanced in lead extraction by the use of several tools and techniques such as locking stylets (Cook Medical and Spectranetics), powered and non-powered sheaths (Evolution®, Cook Medical and TightRail™, Spectranetics) and laser technology53,54 such as the Excimer and GlideLight™ (Spectranetics). An observational retrospective study concluded that lead extraction employing laser sheaths is highly successful with a low procedural complication rate, and that increasing experience is associated with greater success.32 Several reports have described the effectiveness of the Evolution sheath.55–58 The use of locking stylets placed in the lead to facilitate the application of traction and to stabilise the lead during sheath dissection of fibrotic tissue is essential.59 However, the use of the electrosurgical technique for lead extraction seems to be decreasing according to data from the ELECTRA study.48
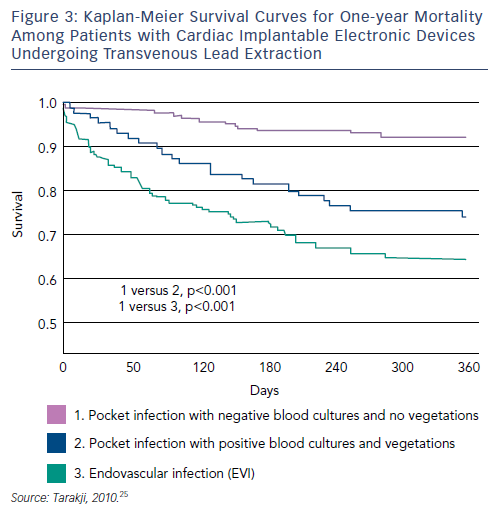
Although TLE intraprocedural mortality is very low, postprocedural and long-term mortality when extraction is performed for the indication of infection remain significant.60–62 A recent study reviewed records of all patients with infected CIEDs who underwent TLE at a tertiary care centre between 2002 and 2008. Patients (n=502) were stratified into two groups: those presenting with pocket infection (n=289, 58 %), and those who presented with bacteraemia, with or without vegetation, and a pocket that appeared benign, termed endovascular infection (EVI) (n=213, 42 %). The one-year mortality rate was 20 %; EVI was associated with significantly higher one-year mortality (HR 2.1, p=0.0008). Among patients with EVI, 100 had vegetation on TOE however there was no difference in one-year mortality between patients with EVI and vegetation compared with patients with EVI and no vegetation. Risk factors for one-year mortality among patients with EVI included chronic renal insufficiency or history of renal insufficiency, end stage renal disease, NYHA functional class III or IV, prior valve surgery, diabetes and bleeding requiring transfusion. The presence of vegetations was not associated with increased one-year mortality (see Figure 3).63
A study of autopsy findings of patients with CIEDs found other issues relating to leads such as thrombi on ventricular/atrial leads (48 %), bipolar lead rings fixed by fibrous tissue (22 %), connective tissue bridges or tunnels in ventricle/atrium (71 %) and ventricular leads fixed to valve or penetrating chordae (46 %).64 Depending on their location, such connective tissue surrounding the leads, as well as leads partially positioned outside the vessels or the heart, may increase the risk of complications during lead extraction. The present HRS and EHRA recommendations do not fully cover the timing of reimplantion or the treatment of pacemaker and ICD dependent patients, partially due to lack of studies comparing strategies. Amendments to the recommendations regarding these issues are highly desirable. Contralateral reimplantation in CIED infection patients is however always recommended, when possible. In summary, advances in TLE have improved procedural safety for patients with CIED infection, but overall mortality remains high and there is a need for further studies to optimise treatment of at-risk patients.
Strategies to Prevent Cardiac Implantable Electronic Device Infection
The first preventive strategy against CIED infection is not reopening a CIED pocket unless necessary. ICD pulse generators in primary prevention systems may not always need replacement. It is possible to maximise battery longevity by setting the lower rate limit (LRL) at 50 bpm, choosing a better LV lead impedance vector for CRT, using devices with quadripolar LV leads or selecting high battery capacity devices, particularly with CRT-D systems. In addition, central lines (vascular catheters) should be avoided in patients with CIED devices, because they may be associated with higher risk of mortality from infection.34
Leadless pacemaker technology provides an alternative that does not require pockets or leads65,66,67 and therefore avoids many of the problems associated with intravascular lead use, including pocket infection. This represents an important therapeutic advance in suitable patients.68 In addition, subcutaneous ICDs (SICD) are now available. The SICD is implanted inferior and lateral to the left breast in mid-axillary line, and the lead is placed under the skin along the left side of the sternum. Therefore the lead is not intravascular or in contact with cardiac tissue, minimising intravascular infection risk.69 However, to date, no comparative studies between the SICD and conventional ICDs have been reported. Therefore, the impact of SICD use on CIED infection rate is not yet known.
Various prophylaxis strategies have been suggested, including skin and nasal infection treatment, device pocket irrigation and operative prep skin barriers, but there is no evidence to support their use in preventing CIED infections. Preoperative cleansing of the patient’s skin with chlorhexidine–alcohol has been found to be superior to cleansing with povidone–iodine for preventing abdominal surgical site infection.70 However, two recent studies showed no significant difference in infection risk among patients undergoing CIED procedure using chlorhexidine–alcohol or povidone–iodine for skin preparation.71,72 In addition, the antimicrobial treatment of pacemaker casings with antiseptics has been investigated in vitro and early studies showed promising results.73
The most common strategy to reduce infections is intravenous prophylaxis using antibiotics.74 A double-blind clinical trial randomised patients (n=649) to prophylactic antibiotics (intravenous administration of 1 g cefazolin immediately before the procedure) or placebo. The trial was terminated early after a significantly lower rate of infection was observed in the antibiotic arm (0.63 % in antibiotic arm versus control [3.28 %]; RR=0.19; p=0.016).74 However, all infections in this study were caused by cefazolin-sensitive isolates and the study population had a low prevalence of methicillin resistance compared with US hospitals (13 % Staph. aureus and 60 % coagulase-negative Staphylococcus species in study versus 55–60 % and 80–90 % in US hospitals).
The use of postoperative antibiotics has been investigated in two recent studies. In a prospective randomised, single-centre study, patients (n=1,008) received standard systemic antibiotic prophylaxis and were then randomised into four groups receiving either povidone-iodine, neomycin, a sterile non-adherent pad or placebo ointment after procedure. All patients were followed for at least 12 months. Surgical site inflammation and infection were graded based on degree of inflammation, discharge, wound culture and blood culture. The surgical site infection rate was more than doubled in those with longer procedural time (HR=2.3, p=0.01) but the use of topical antibiotics after closure did not show significant benefit.75 A prospective database on patients undergoing PPM implantation from 1991–2009 (n=3,253) found that over 19 years the incidence of CIED infections fell from 3.6 % with no antibiotics to 2.9 % (perioperative antibiotics), to 0.4 % (peri- plus postoperative antibiotics), suggesting that perioperative followed by postoperative antibiotics may minimise infections.76 However, the REPLACE registry found no difference in infection rate between those who received postoperative antibiotics and those who did not.6
The Prevention of Arrhythmia Device Infection Trial (PADIT) clinical trial is currently recruiting and aims to compare a centre-wide policy of incremental antibiotic therapy with conventional antibiotic prophylaxis in high-risk patients undergoing CIED implantation. Centres (not patients) will be randomised to either conventional antibiotic therapy (cefazolin or vancomycin for penicillin-allergic patients) or incremental antibiotic therapy comprising a single preoperative dose of cefazolin and vancomycin (vancomycin only in patients allergic to penicillin), an intraoperative bacitracin pocket wash then two days of postoperative antibiotic therapy comprising cefalexin or cephadroxil (clindamycin in penicillin-allergic patients). Centres will be randomised to one therapy and then crossover after 6, 12 and 18 months. During each treatment period the randomised antibiotic therapy will be used on all patients undergoing a device implant procedure.77,78
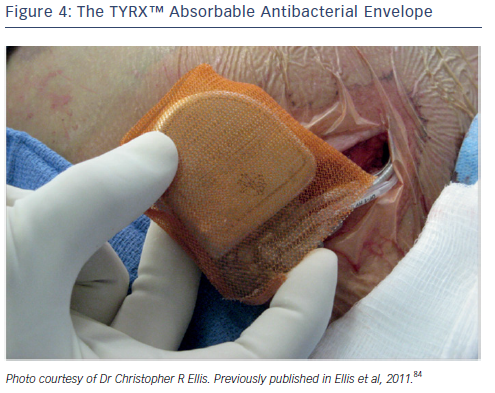
An additional strategy to combat pocket infection involves the use of an antibiotic envelope. The TYRX™ non-absorbable envelope (Medtronic) has shown substantial efficacy in clinical studies in reducing the infection rate.23,79,80 This has led to the development of the TYRX absorbable envelope, which is constructed from a fully bioabsorbable multifilament mesh (see Figure 4). The envelope holds the CIED in place, preventing device migration, elutes antimicrobial agents minocycline and rifampicin for a minimum of 7 days; and then is fully absorbed approximately 9 weeks after implantation. The TYRX envelope received US Food and Drug Administration clearance in May 2013 and the CE Mark in September 2014.
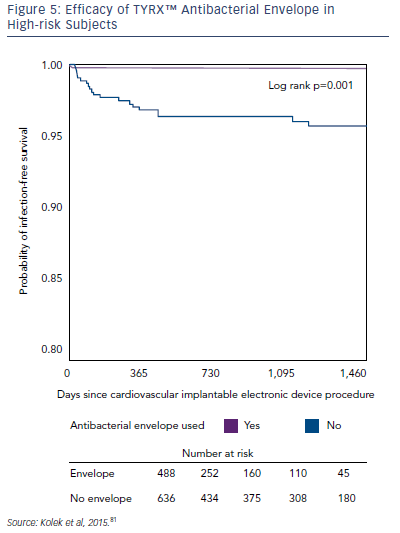
A growing body of evidence has demonstrated the efficacy of the TYRX envelopes in the prevention of CIED infections. In a singlecentre retrospective cohort study, the infection rate in patients with ≥2 risk factors for CIED infection was compared in patients receiving the TYRX absorbable envelope (n=135), the TYRX non-absorbable envelope (n=353) and controls who did not receive an envelope (n=636). After a minimum 300 days, CIED infections were reported in 0 % of patients receiving the absorbable TYRX, 0.3 % for the nonabsorbable TYRX, and 3.1 % for controls (p=1 for absorbable versus non-absorbable TYRX; p=0.03 for absorbable TYRX versus controls, and p=0.002 for non-absorbable TYRX versus controls; see Figure 5). This represents a very low prevalence of infection in subjects at risk and suggests that the use of the TYRX absorbable antibacterial envelope is a promising strategy.81
Two large (n=1,129) prospective multicentre cohort studies are currently investigating the impact of the TYRX non-absorbable envelope on CIED major infections and mechanical complication rates. The Citadel (TYRX Envelope for Prevention of Infection Following Replacement with an Implantable Cardioverter-Defibrillator) and Centurion (TYRX Envelope for Prevention of Infection Following Replacement with a Cardiac Resynchronisation Therapy Device) studies aim to compare the rate of CIED infection and mechanical complication after CIED replacement with an ICD or CRT. Recently presented data indicated that the TYRX antibacterial envelope reduces the infection rate by 80 % compared with historical control data.82 However, it should be noted that the comparison of data with historical controls has well-known limitations, and there is a need for randomised study data to confirm the effect of the TYRX envelope.
The Worldwide Randomised Antibiotic Envelope Infection Prevention Trial (WRAP-IT) is a multicentre, single-blinded, randomised study that aims to evaluate the ability of the TYRX absorbable antibiotic envelope to reduce major CIED infections during 12 months following CIED generator replacement, upgrade, revision or de novo CRT-D implant.78 Patients (around 7,000) will be randomised 1:1 to envelope versus no envelope. The primary endpoint is the rate of major CIED infections leading to one or more of the following: CIED system removal, CIED pocket revision, antibiotic therapy or death. Secondary objectives include all cause mortality and CIED removal due to pain without obvious infection. The study also aims to determine the one-year incidence rate of CIED infection among a large cohort of patients undergoing CIED procedures, as well as elucidating risk factors for CIED infection.
A recent retrospective study analysed data from patients who underwent CIED implantations, with (n=365) or without (n=1,111) the TYRX envelope. In the non-TYRX group, 19 infections were observed (1.7 %), versus 0 in the TYRX group (p=0.006). It was estimated that the TYRX prevented 6.2 additional infections costing approximately $340,000. This cost was similar to the actual cost of the envelopes in the TYRX group, estimated at $320,000.83 Therefore use of an antibacterial envelope as standard care appears to be economically reasonable.
Discussion
As a result of the increasing incidence and complexity of CIED treatment, infection is frequently encountered in clinical practice and is associated with significant morbidity and mortality. Moreover, the infection rate is rising faster than the rate of CIED implantation. Many questions remain unanswered, including the true infection incidence, clear infection definitions, better understanding of risk factors and the impact of implantation practices and techniques.
More experience and advances in TLE including laser technology and rotational sheaths have reduced procedural complications; however device infection, despite lead extraction, is associated with long-term mortality of 15–25 % at one year. The mortality risk is higher with endovascular infection than with pocket infection.
There is a need for further studies aimed at elucidating the complication and mortality risks associated with device and lead extraction: these may help guide CIED and lead management as well as extraction tool and technique development.
In terms of preventing CIED infections, interim data from nonrandomised studies indicate that the use of the TYRX antibacterial envelope appears to be one promising and cost-effective strategy in preventing CIED infections. The WRAP IT study will help assess the efficacy of the absorbable TYRX envelope in reducing infection in a prospective randomised large clinical trial.