This study reviews how haemodynamic monitoring devices that provide indirect or direct measures of heart failure status relate to cardiac rhythm management devices for the management of patients with the condition. The role of patient-facing software and services that provide information from these devices to patients can create a new model of heart failure disease management. In this care paradigm, patients are provided with daily data that can be used to aid in self-management and provide exception-based data to physicians and care teams, allowing them to care for more patients more efficiently.1
Cardiac Rhythm Management Devices in Heart Failure
Cardiac rhythm management devices, consisting of ICDs and resynchronisation devices, are routinely implanted in patients with heart failure for primary prevention of sudden cardiac death, as well as for the treatment of heart failure. In the US, roughly 100,000 ICDs are implanted each year.1 Based on current guidelines, ICD therapy is indicated in patients with left ventricular ejection fraction (LVEF) ≤35 % who are at least 40 days post myocardial infarction and have functional class II or III heart failure.2 Similarly, cardiac resynchronisation therapy is indicated for patients with LVEF ≤35 % in sinus rhythm with a QRS duration >0.12 milliseconds and functional class III–IV heart failure on optimised medical therapy.2
Heart Failure: A Long-term, Costly Condition
Heart failure treatment costs are estimated to be more than $30 billion each year in the US.3 These costs pay for healthcare services such as ambulatory visits, admissions, procedures and pharmacologic therapy.3 The prevalence of heart failure is increasing and it is a long-term condition characterised by disease progression that is associated with a 30 % 5-year mortality rate.4
Gaining a deeper understanding of how to support patients with heart failure by developing a more holistic and continuous care model that considers every aspect of disease management and involves the patient and gives them tools to self-manage this complex, chronic condition is needed. Current consumer and FDA-regulated hardware, software and services that are already used by billions of consumers can be harnessed to serve this model. However, additional solutions need to be developed that empower and provide continuous support and education for patients with heart failure, as well as their personal care network, and facilitate communication between caregivers and provider teams.5
What Defines Haemodynamic Monitoring in Heart Failure?
Heart failure progression and haemodynamic decompensation, most commonly manifesting as increased intracardiac pressure due to volume overload, are predictive of adverse events and mortality. Active ambulatory surveillance of intracardiac pressures, using indirect correlative or direct measurements, can guide early treatment intervention and reduce costs, hospitalisation and mortality.6–17
One of the first indirect assessments of haemodynamic status that can be measured from a cardiac rhythm monitoring device uses impedance measurements as a surrogate for intrathoracic impedance. Measured by the implanted device between the right ventricle lead and the pulse generator, impedance reductions correlate with increases in thoracic fluid volume. The utility of this measurement as an early indicator of heart failure decompensation is based on data showing that impedance reductions can be detected up to two weeks before a patient develops symptoms of heart failure decompensation.9
In a trial that compared device measured impedance changes to daily weight gain (the Fluid Accumulation Status Trial), intrathoracic impedance was reported to be a more sensitive and an earlier indicator for detecting heart failure events.10 However, there are significant limitations associated with using impedance changes to guide clinical decision making. Patients have no access to the measurements and it is incumbent upon the remote monitoring clinic to follow and evaluate the clinical significance of an impedance change. There is also a significant false positive rate of 1.5 detections per patient-year of follow-up.9 Causes of false-positive readings include lung disease or infection, pocket haematoma and body type (habitus).11
The recently completed Multisensor Chronic Evaluation in Ambulatory Heart Failure Patients (Multi-SENSE) trial evaluated and validated multiple device-based measurements of heart failure status in patients with cardiac resynchronisation therapy devices. The study prospectively evaluated a combination of intrathoracic impedance, heart and respiratory rate, heart sounds and activity. A proprietary composite algorithm based on these variables predicted heart failure events up to 34 days before patient presentation with 70 % sensitivity and 87.5 % specificity.12 While more accurate than thoracic impedance alone for predicting heart failure events, this algorithm lacks patient engagement or a defined care pathway for evaluating patients whose measurements indicate their heart failure is worsening. Furthermore, this is not a device that an implanting electrophysiologist or implantable device follow-up clinic is set up to manage. At most centres, the device follow-up care team reviewing ICD remote data is not the team monitoring patients’ heart failure status and titrating medications or recommending intervention.
Nevertheless, trends in patient symptoms or activity can be collected using consumer devices, such as fitness trackers or smartphones with embedded activity sensors, and these can be used in the management of heart failure. This is because they measure functional status continuously and provide personalised data profiles of individual heart failure patients over time.5
Direct measurements of remotely collected intracardiac pressures present a clear and clinically actionable targets for intervention. There is an FDA-approved pulmonary artery pressure sensor that is indicated and labelled for this purpose. This device measures pulmonary artery pressure from a battery-free electromechanical sensor, which is implanted using a minimally invasive over-the-wire technique in the distal pulmonary artery.13–15 Daily ambulatory pressures remotely collected from the sensor are used to direct heart failure medical therapy.
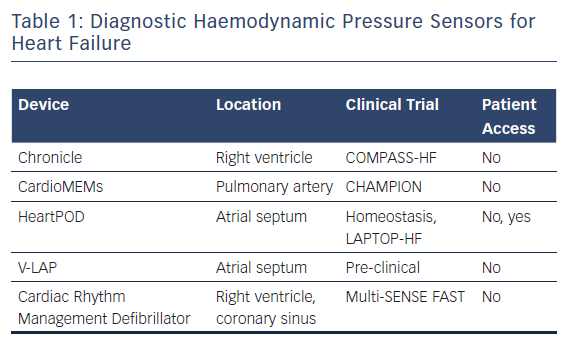
The CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial assessed the rate of heart failure-related hospitalisations at 6 months in ambulatory class III heart failure patients who had been hospitalised within the previous 12 months. Patients were randomly assigned to pressure-directed heart failure therapy versus standard heart failure management. Those randomised to pressure-directed therapy experienced a 28 % reduction in hospitalisations related to heart failure.14 Post-FDA approval registries have also established long-term reductions in heart failure events and costs associated with the condition. These data show that patients with heart failure benefit outside a clinical trial and that this benefit extends over longer follow-up intervals that were studied in the clinical trial.15,16 Like those enrolled in trials of indirect measurements of heart failure status, in the CHAMPION study, patients were not given their pressure readings (Table 1). Instead, the information was provided to the care team on a secure website; it was reviewed at least weekly and guided recommended adjustments in medical management were provided to the patients.
An earlier device, which measures right ventricular pressure with a right ventricular pressure-sensing lead, demonstrated that right ventricular pressure correlates with pulmonary artery diastolic pressure and that pressure excursions preceded heart failure clinical events. However, when studied in a clinical trial – the COMPASS-HF trial – the trial did not meet its pre-specified endpoints. This was attributed to a lack of aggressive management to reduce elevated pulmonary pressures as well as unexpectedly better outcomes than anticipated in patients not randomised to the sensor.17
Another haemodynamic pressure sensor, extensively studied in the Homeostasis and LAPTOP–HF trials, directly measures left atrial pressure using a trans-septal sensor. In these studies, patients were provided with a handheld device that recorded sensor readings and allowed them access to pressure tracings that were collected twice daily and wirelessly communicated to their heart failure care team. Patients were remotely prescribed pharmacological therapies via the handheld device, based on a preset algorithm that directed therapy based upon pressure ranges.
The LAPTOP-HF trial was a prospective, multicentre, randomised study that enrolled patients with class III heart failure who had been admitted to hospital for heart failure within the past year. It defined safety as freedom from major adverse cardiovascular and neurological events within 12 months, and efficacy as a reduction in heart failure hospitalisations and all-cause mortality. Unfortunately, the data safety and monitoring board halted the trial against the recommendations of the study investigator leadership team after more than 500 of the planned 730 patients were enrolled because of a temporal clustering of transseptal related procedural complications. Patients continued to be followed after the trial was halted for the study endpoints of hospitalisation and mortality. Retrospective analysis of the outcome data revealed that the safety boundaries of the trial had not been crossed and that the patients who were managed with medical therapy, directed by left atrial pressure measures, had a 41 % reduction in heart failure hospitalisation at 12 months’ follow-up.17 These results support the concept that patients can be provided with their own haemodynamic data that allows them to work effectively in partnership with their treatment team in their own heart failure care. Despite these results, the sponsor did not seek FDA approval, as statistical power to detect a treatment difference was not achieved because the trial had been terminated prematurely.
Another left atrial pressure sensor is in early preclinical testing. The V-LAP device is a left atrial leafless and wireless sensor that is implanted in the septum. Similar to the sensor used in the LAPTOP–HF trial, the V-LAP sensor records an atrial ECG and provides left atrial waveform morphology, potentially allowing other comorbidities such as valvular regurgitation and arrhythmias to be detected.18,19
Devices that Improve Haemodynamics
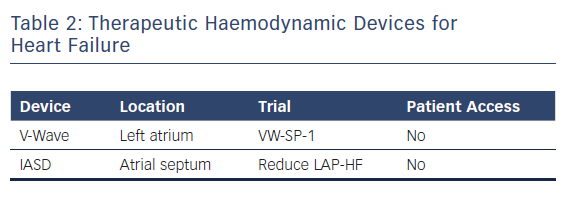
A new generation of atrial shunt devices that aim to reduce left atrial pressure but do not incorporate implantable haemodynamic sensors is under investigation. Two devices are being studied in pivotal clinical trials and both work by creating atrial septal communications to allow for left to right atrial flow to reduce left atrial pressure.20–22 Both have shown safety in feasibility studies and have had a positive impact on cardiac haemodynamics in phase I and II trials (Table 2).20–22
End Stage Heart Failure and Haemodynamic Devices
Of the 6 million people diagnosed with heart failure, roughly 250,000 have progressed to advanced disease (stage D), which is characterised by frequent admissions, inotrope use or mechanical circulatory support.6
In these cases, because of the advanced stage of the disease, neither cardiac rhythm monitoring device therapies, such as resynchronisation, nor diagnostic sensors are efficacious. These patients have mortality rates of over 50 % in 6 months.22–25 Because too few donor hearts are available (2,000 donors per 100,000 potential candidates), ineligibility for transplant or clinical instability, many patients are treated with durable or permanent left ventricular assist devices (LVADs).26,27 In these patients, right and left heart failure is an acute and chronic complication of assist device therapy and heart failure is a frequent cause of hospital admission after device implant.27–29
Implantable pulmonary artery sensors are not well studied in this population but may have utility both to predict early eligibility for assist device before decompensation or instability and to prevent heart failure events after implant. A subgroup analysis from the CHAMPION trial showed that patients with a pulmonary artery pressure sensor who went on to require LVADs had more medication changes and were referred for and underwent transplantation earlier than those without a sensor.28
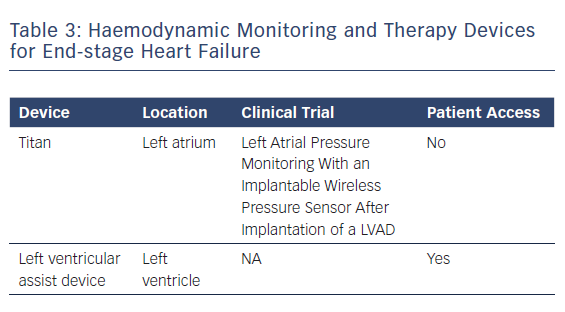
A recently published case study reported the use of a wireless pressure sensor for monitoring left atrial pressure during LVAD support (Table 3). The sensor is designed to guide pump speed, help manage intravascular volume and tailor the use of medications such as intravenous inotropes. Integrating haemodynamic pressure sensors into LVAD technology is an attractive strategy.29
Patient-Facing Data and Tools for All Heart Failure
Management of heart failure across the disease spectrum includes pharmacological therapy, device implantation, patient education and clinic visits. It is clear that adherence to treatment plans slow down disease progression.6
However, compliance to treatment regimens and patient understanding and ability to manage their condition, especially before decompensation events, is suboptimal.30 For many patients with heart failure, drug regimens can include up to six medications, some scheduled up to three-times per day. Patients must also adhere to salt-restricted diets and observe fluid restrictions. While several studies have attempted to provide patients with a continuing model of disease management support outside hospital, little improvement has been made in reducing event rates with nurse phone calls or intermittent home visits.30,31
Cardiac rhythm devices and haemodynamic monitoring data provide crucial information to the treating physician. This continuous ambulatory data guides dynamic changes to medical therapy and improves all-cause outcomes. Given the daily fluctuations in heart failure haemodynamics, providing patients with objective data collected from these sensors can engage them in their own care and encourage them to work more closely with their care team and take a more active role in their disease management.2,5
However, most wireless monitoring allows only physician access and patients are kept blinded to their own pressure tracings or rhythm monitoring. This denial of data is completely out of sync with today’s culture of on-demand access to media, entertainment and financial data. For example, more than one billion people around the world have access to internet-connected phones and most use their smartphone as the primary method for internet search.32,33 Supporting this further, over 50 % of patients seek remote medical care.34 Apart from smartphone technology, wearable sensing devices that are designed to track metrics such as activity, calories spent and heart rate are overwhelmingly popular.35 Reports show that up to 25 % of Americans use a wearable sensor.34 These data suggest that patients want to feel empowered and directly involved in their health by implementing digital technology tools, which are easily available.
With increasing patient interest and today’s connected medical technology, digital disease management has the potential to change the face of heart failure diagnosis and treatment. Continuous tracking of cardiac haemodynamics, medication adherence, activity and diet are perfectly suited to a digital model of care specific to the patient’s condition where diuretics can be increased, goal-directed medical therapy can be continuously optimised and lifestyle adjustments can be recommended.5,34 Software solutions can also provide a platform for asynchronous communication between patients, caregivers and providers. This allows for seamless data sharing, caregiver efficiency and a continuous model of patient-supported care.34,35 This is crucial in long-term conditions, such as heart failure where gaps in care and communication can lead to progression of the disease and adverse outcomes.
The first smartphone-compatible implantable cardiac rhythm monitor has recently been approved by the FDA.36 This software app allows patients to interrogate their device and transmit data securely to their treating physician. Engaging the patient further by letting them view their continuous ECG and providing activity and other information that the smartphone can collect is a natural extension of this product’s capability that should be explored.
Conclusion
While disease progression is inevitable for most patients with heart failure, outcomes can be improved with continuous monitoring and dynamic therapy adjustments. Recent digital technology such as pressure sensors and cardiac rhythm management devices have been proven to predict heart failure events and reduce hospitalisations.37–39
Most of these devices transmit data only to treatment teams and patients are left unaware of their own personal health information. Patients are increasingly becoming more involved in tracking their own health using digital hardware and software, including downloading healthcare apps on their smartphones or investing in wearable sensors. Consumer and chronic care populations are already using wearable devices and devices such as mobile ECGs to monitor activity and health status.40
These new digital care models of heart failure management need to be prospectively studied and clinical workflows need to evolve to properly manage patients more continuously and efficiently.
Protection of devices and data flow are paramount to patient trust in these solutions and regulations regarding cybersecurity.41 Nonetheless, providing patient-facing data and decision support for people with heart failure using digital healthcare and software tools should be a priority for research and validation.
Clinical Perspective
- Haemodynamic monitoring represents the next era in heart failure care.
- Digital health software and services can help the patient and physician manage heart failure more continuously using more personalised data.
- Digital tools, including those that use data from implantable devices, will support disease management for patients with heart failure.
- These tools are patient facing and should be studied.
- New clinical workflows, including digital patient services and exception-based management require study and development.