Since the 1960s, Holter monitoring has been a cornerstone for diagnosing suspected arrhythmias in patients of all ages.1 The most common monitoring systems allow the continuous registration of three or more leads for 24–48 hours; newer Holter monitors allow continuous electrocardiogram (ECG) registration for 2 weeks.1 Extending the time of ECG registration will increase the diagnostic yield of Holter monitoring, especially for those rhythm disturbances that are infrequent but recurrent.1,2 This need for a prolonged ECG monitoring has been addressed by event recorders, which can monitor patients for up to 3 years, storing the ECG obtained a few minutes before and after the onset of an arrhythmia in its memory and transmitting data to the cardiac unit.1,2
When interpreting the results of the ECG, the cardiologist has to determine whether symptoms reported by the patient could be linked to significant disturbances in heart rhythm.2 In other circumstances the detection of atrial or ventricular arrhythmias may alert the cardiologist, even if they occur asymptomatically, thus prompting a specific therapeutic decision such as starting antiarrhythmic or anticoagulant drugs or implanting a pacemaker or a cardioverter defibrillator.2
Cardiac Monitoring Systems
Cardiac rhythm monitoring has an established diagnostic and prognostic role in different circumstances: syncope, palpitations and monitoring of patients with known or suspected episodes of atrial fibrillation(AF), e.g. those with stroke of uncertain aetiology (cryptogenic stroke).1–3 ECG monitoring may also play a role in identifying ventricular tachycardia (VT) in patients with recognised risk of sudden cardiac death.4 As many devices with different characteristics are available, the choice of the most suitable monitoring system largely depends on the likelihood of detecting a significant correlation between symptoms and ECG findings.1
Holter Monitors
Traditional ambulatory Holter monitors are simple devices that usually have three leads and continuously register the ECG. They can be short- (24–48 hours) or long-duration (1–2 weeks) devices.1 Two-week Holter monitoring is preferred when diagnosing or excluding AF in cryptogenic stroke, but loop recorders and outpatient telemetry have even higher detection rates.1
There are two main advantages of a continuous Holter monitoring system: the possibility of quantifying the real burden of an arrhythmia, and the detection of rhythm disturbances outside the limits set by an algorithm or memory. The quantification of arrhythmic events may aid the clinician in making a therapeutic decision, especially for those arrhythmias that occur frequently and those that have disabling symptoms. Despite their advantages, ambulatory Holter monitors also have many limits: a relatively brief duration of monitoring, the impossibility of transmitting real-time data to the attending cardiac unit and the need for close collaboration between the patient and health professionals.
Loop Recorders and Post-event Recorders
Newer generation monitors are the so-called event recorders. According to their specific functions they can be divided into two categories: loop recorders, which include external loop recorders (ELRs) and implantable loop recorders (ILRs); and post-event recorders (non-looping recorders).1
Loop recorders are event recorders with a ‘loop memory’: they continually analyse the ECG and retain information pertaining to relevant arrhythmias that are automatically detected thanks to predefined algorithms and the registration of the ECG a few minutes before the onset of the arrhythmia.1,2 As event recorders can be activated by the patient when he or she experiences symptoms, they can reliably document a correlation between symptoms and an arrhythmia, as well as excluding a causative role of heart rhythm disturbances in determining syncope or palpitations when such symptoms occur without any arrhythmia (class I, level B evidence).2
ELRs can monitor the ECG for a maximum of 30 days.1,2 An ELR can be connected to a belt around the chest, without the need for traditional electrodes.5
ILRs are small devices that are designed for subcutaneous implantation in the chest wall via a minimally-invasive surgical procedure.3 The preferred site for implantation is the left parasternal area of the chest, even if the ILR can be placed in other locations, e.g. the left axilla, the inframammary region and the space between the supraclavicular notch and the left breast area.3 They can be fixed to the chest wall in order to reduce the number of artefacts on the ECG signal that are attributable to the mechanical instability of the device.3 Despite higher costs, ILRs are safe, have a low rate of infection (2–4 %)6 and can continue monitoring for up to 3 years.2 They may also be useful for noncompliant patients, as there are no external parts to be worn.3 Like other event recorders, ILRs are designed to transmit data to a distant diagnostic station.1,2 This remote monitoring function can be automatic, via the internet, or on demand, with the patient being required to activate telephonic transmission of the ECG data. Data transmission is simple, and patients can be trained to do this.1,2 Remote monitoring may prompt intervention and therapy if a clinically relevant arrhythmia is detected. ILRs allow the registration of only one lead, rendering the interpretation of the ECG difficult in some cases.1–3 Moreover, they have limited storage capability (generally less than 1 hour) and thus some arrhythmias may be missed if they are very frequent.1,2 Most currently used ILRs are compatible with MRI, however the device’s technical manual should be carefully consulted to determine whether such imaging is safe. If directed towards the device, sources of radiation for both diagnostic, e.g. CT, and therapeutic purposes may impair its function.
Post-event recorders can be used for 14–30 days.1 The monitoring function starts when the patient puts the device on his or her chest as symptoms commence. For this reason, the diagnostic yield of post-event recorders is limited by the potential loss of events causing disabling symptoms that prevent patients from activating the device.1,3 There is also the risk that patients may forget to activate the device. Some post-event recorders have an extended backward memory, e.g. 15 minutes, which allows time to handle the patient before pressing the button to save a recording.7
Mobile Cardiac Outpatient Telemetry
To overcome many limits of the event recorders, mobile or real-time cardiac outpatient telemetry (MCOT) systems have been developed.1 They are external ambulatory monitors that can monitor patients for up to 30 days.1 Their continuous analysis of the ECG and realtime transmission of every single event to the attending cardiac unit gives them an advantage over ELRs.1 It is estimated that traditional 24–48-hour Holter monitors have a diagnostic yield of 15–28 %,8–10 while ELRs have a yield of up to 63 %.11 In a randomised controlled trial of 266 patients with presyncope, syncope or severe palpitations, MCOT allowed a diagnosis in 41 % of patients, while the ELR arm had a diagnosis in only 15 % of cases (P<0.001).12
Loop Recorders in Syncope and Palpitations
The European Society of Cardiology gives a class I indication for ILRs in early phase evaluation in patients with recurrent syncope of uncertain origin and ELRs in patients with recurrent palpitations when conventional ECG monitoring has not established a diagnosis.2 When palpitations are severe and infrequent, with an inter-symptom interval >4 weeks, or ELR findings are inconclusive, the use of an ILR may be indicated (class IIA).2 The rationale for these recommendations is that syncope usually occurs less frequently than palpitations, making an ILR more suitable for an early diagnosis when one or more 24-hour Holter monitoring sessions have been negative despite the recurrence of symptoms.2
Devices with a maximal monitoring duration of 1 month, such as ELRs, can diagnose most rhythm disturbances causing palpitations when symptoms occur at least monthly.2 Locati and colleagues reported a similar diagnostic yield over 1 month between ELRs and ILRs in presyncope, syncope and palpitations.4 The authors concluded that an ELR may be indicated for the initial screening of patients with recurrent unexplained syncope or presyncope in place of a more expensive ILR, which remains an option if, after 1 month, monitoring with an ELR has proven negative.4
Data from the Place of Reveal in the Care Pathway and Treatment of Patients with Unexplained Recurrent Syncope (PICTURE) registry on 570 patients with unexplained recurrent syncope show that in 1 year, 36 % of patients with syncope of unknown origin are expected to have symptoms and ILRs can guide the diagnosis in up to 78 % of events, even when traditional diagnostic tests (including ambulatory ECG monitoring) have failed.13 The International Study on Syncope of Uncertain Etiology (ISSUE-1) study showed that the majority of syncopal events documented with an ILR is likely to be neurally mediated, with asystolic pauses and reflex bradycardia being the most frequently associated rhythm disturbances.14 Loop recorders effectively guide therapeutic decisions: data gathered from the prospective multicentre observational study ISSUE-2 show that therapy including pacemaker or implantable cardioverter defibrillator (ICD), anti-arrhythmic drug therapy and catheter ablation guided by ILR findings leads to a significant reduction in the number of symptoms per year (92 % relative risk reduction), making ILRs suitable for the early management of patients with recurrent unexplained syncope of suspected neurally mediated origin.15 The multicentre randomised controlled ISSUE-3 trial on pacing therapy for asystolic neurally mediated syncope confirms that ILRs effectively guide treatment decisions, with a 57 % reduction in syncope recurrence when dual-chamber permanent pacing is adopted as compared with no pacing.16 Reports from Farwell and colleagues extend the high diagnostic value of ILRs to all patients with unexplained recurrent syncope, not only those with neurally mediated syncope.17
In our experience on a heterogeneous cohort of patients with syncope or presyncope and negative extensive evaluation including 24-hour Holter monitoring or in-hospital telemetry, an ILR-guided diagnosis was obtained in about half of cases. The most frequent pathogenic mechanisms were bradycardia, asystole and advanced atrioventricular block, with tachycardia accounting for a minority of cases (see Figures 1 and 2). ILR findings prompted the implantation of a pacemaker or an electrophysiological study and specific therapy (transcatheter ablation or implantation of a cardioverter defibrillator) in patients with documented arrhythmic syncope.18
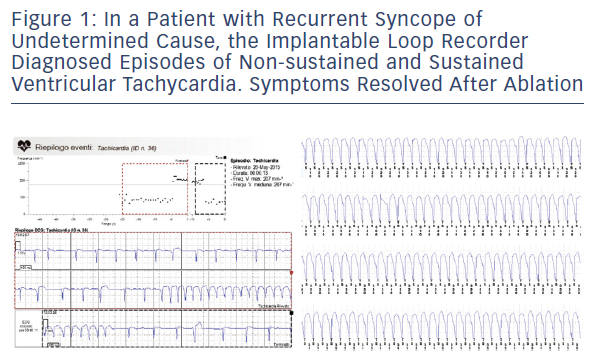
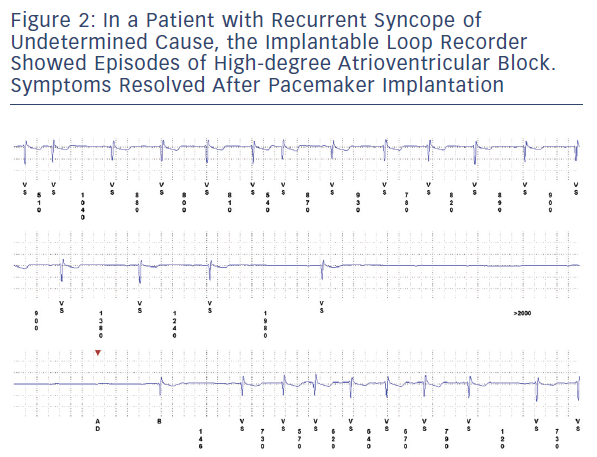
Apart from well-established indications in the diagnosis of cardiac causes of syncope, presyncope and palpitations, there is growing evidence that loop recorders may play a role in the management of suspected AF and in the risk stratification of patients with structural heart disease.2,3 It is likely that future guidelines on the use of loop recorders will encompass these clinical situations, in light of recent evidence.
Possible Contraindications
There are some patients in whom the use of an event or loop recorder as initial diagnostic tool may be contraindicated in light of an estimated high risk of a life-threatening arrhythmia. Patients at high risk are those with an indication for a pacemaker or ICD independent of the diagnosis of the cause of syncope, those with structural (e.g. heart failure) or coronary heart disease, those with clinical and ECG findings that drive suspicion for an arrhythmic syncope, and patients with important comorbidities.2 When one or more of these characteristics are present, the recommended strategy is hospitalisation for treatment or extensive evaluation including in-hospital prolonged telemetric monitoring or electrophysiological study.2 An ILR becomes an option after an extensive diagnostic work-up has proven negative.2 The same recommendations apply to high-risk patients with palpitations.2
Loop Recorders in Cryptogenic Stroke
AF has a prevalence of 1–2 % in the general population and up to 9–10 % in people aged over 65 years.19 The major risks associated with unknown AF, both persistent and paroxysmal, are ischaemic stroke and other thromboembolic events, which could be prevented by a prompt diagnosis of AF and consequent anticoagulant therapy.20,21 As there is poor correlation between the occurrence of AF and symptoms, an ECG monitoring strategy is needed when paroxysmal AF is suspected.20,21 This is the case for patients with cryptogenic stroke (stroke with unknown aetiology after thorough evaluation), in whom AF episodes most often occur asymptomatically.22 In 20–40 % of patients, the cause of ischaemic stroke cannot be determined after a complete diagnostic evaluation including ambulatory Holter monitoring for 24 hours or longer.23–27
Two multicentre randomised controlled trials tested the hypothesis that loop recorders increase the number of patients correctly diagnosed as having AF, potentially sparing a significant number of recurrent strokes. Investigators from the 30-Day Cardiac Event Monitor Belt for Recording Atrial Fibrillation after a Cerebral Ischemic Event (EMBRACE) trial showed that an ELR strategy improves the detection rate of AF over a traditional approach of 24 hours of continuous Holter monitoring in cryptogenic stroke and transient ischaemic attacks of undetermined cause.5 AF lasting 30 seconds or longer was detected after 30 days in 45 out of 280 patients (16.1 %) with the use of an ELR (ER910AF Cardiac Event Monitor, Braemar Inc), against a detection rate of nine out of 277 (3.2 %) in the control group (P<0.001). The authors estimated that for every eight patients assigned to the ELR, one would be diagnosed as having AF despite negative 24-hour ECG monitoring.
Additional data in favour of the early use of loop recorders in the diagnosis of AF as cause of cryptogenic stroke come from the Cryptogenic Stroke and Underlying (CRYSTAL) AF trial.22 Four-hundredand- forty-one patients aged 40 years or more with a diagnosis of cryptogenic stroke or transient ischemic attacks of undetermined cause were randomly assigned to an ILR (Reveal XT, Medtronic Inc) or a conventional ECG monitoring strategy. The follow-up was completed at 12 months, with the primary endpoint being the time to first detection of AF lasting 30 seconds or longer at 6 months. AF was diagnosed at 6 months in 19 (8.9 %) of the 221 patients randomised to the ILR compared with three patients (1.4 %) in the control group (P<0.001). The majority of AF episodes occurred in the first 6 months after randomisation, but the diagnostic yield of the ILR accrued until the end of the follow-up period. By 12 months, AF had been correctly diagnosed in 29 patients (12.4 %) in the ILR group and only four patients (2 %) in the control group (P<0.001).
An observational study on 60 patients with cryptogenic stroke showed that ILRs might have a higher diagnostic yield than ambulatory Holter monitoring lasting 7 days.28 This finding is consistent with results from the CRYSTAL AF trial, in which the mean time between randomisation and the first episode of AF was longer than a week (38 ± 28 days).22
The AF detection rate in the CRYSTAL AF trial was lower than in the EMBRACE trial, even though the follow-up was considerably longer (12 months compared with 1 month). However, the population studied in the CRYSTAL AF trial was younger and had a lower prevalence of hypertension: two baseline characteristics associated with a reduced risk of AF.19,29
Loop recorders such as ER910AF and Reveal XT are programmed to automatically detect episodes of AF using algorithms based on the irregularity of the R-R interval.5,22 They are also capable of remote transmission of data. For example, the transmission of data registered by the Reveal XT can be accomplished through a CareLink Network.22
In consideration of the high diagnostic yield of AF-detecting ELRs as shown by the EMBRACE investigators, we believe that an ELR could be used as first-line monitoring strategy in cryptogenic stroke after an initial extensive evaluation including 24–48-hour ambulatory Holter monitoring has been negative. In our opinion, the more expensive ILRs remain a valuable option for long-term monitoring in a stepwise approach to patients with cryptogenic stroke with high suspicion of AF despite negative 30-day follow-up with an ELR.
Holter Monitors and Loop Recorders in Patient Follow-Up
Ambulatory Holter monitoring for 24 hours or more is useful in the follow-up of patients with persistent or permanent AF in whom a rate-control strategy has been adopted, allowing for optimisation of therapy with normal heart rate as the target.1 New-generation ILRs with algorithms for the detection of AF are being used in research and in clinical practice to monitor patients after transcatheter ablation of AF.30 About 40 % of 129 patients that had pulmonary vein isolation with radiofrequency for rhythm control had recurrence of AF within 1 year from the procedure, as documented by the ILR Reveal XT.31 Results of the cryoballoon isolation of pulmonary veins are comparable.32 These data are consistent with a meta-analysis of 63 studies that shows a recurrence rate of 43 % after a single procedure and of 29 % after repeated procedure.33 Detection of recurrent AF requires a second ablation or a change in therapeutic strategy (e.g. rate control), making ILRs very important in treatment decisions for AF patients.20,21,30
Current guidelines recommend at least a 2-year follow-up after catheter ablation, with repeated ECGs and ECG monitoring.30 ECG monitoring strategies include short- and long-term ambulatory Holter monitoring, ELRs, MCOT systems and ILRs.30 ILRs may identify AF recurrences when they are very infrequent and ILRs are suitable for long-term follow-up of patients whose therapy is driven by silent AF detection, e.g. the decision to give anticoagulation to patients with thromboembolic risk factors.30 There is some evidence that the amount of arrhythmia in AF patients correlates with thromboembolic risk, but a large study is required to validate this finding.34 The arrhythmic burden can be quantified with a continuous monitoring system, even though ILR data have shown a good correlation with continuous Holter monitoring.35,36
Finally, the registration of the beginning of an AF episode with event recorders may help to establish the pathogenic mechanism of the arrhythmia. For example, AF episodes that are preceded by frequent atrial ectopic activity most likely have a focal origin.37 In this case, pulmonary vein isolation with transcatheter ablation should be expected to halt the arrhythmia.37
Smartphone ECG Monitoring
A novel technology that has shown promising results in the monitoring of patients with AF is smartphone-enabled ECG, like AliveCor (AliveCor Inc).38 The AliveCor is a small device containing two electrodes that can turn a smartphone into a single-lead ECG. This system has been validated with 12-lead ECG, is not costly and allows for remote transmission of data.39 Pilot studies report a good correlation with standard ECGs in diagnosing AF and supraventricular tachycardia, but results are preliminary.40,41 Of interest, there was high acceptance of this diagnostic tool by patients,40 so it may have a future role in the screening of arrhythmias in symptomatic patients.39 Cardiac monitoring with the AliveCor system is intermittent and can be performed several times per day with continuous recording for up to 5 minutes. For this reason it could be especially useful in diagnosing symptomatic arrhythmias.
Algorithms and Diagnostic Accuracy of ILRs
The main diagnostic issue with ILRs is that they have ECG storage limits and artefacts can potentially overwrite real arrhythmias. However, the detection algorithms of ILRs have been extensively validated using continuous Holter monitoring as the gold standard.35,36 In the Reveal XT Performance Trial (XPECT), the detection algorithm of the Reveal XT, which is based on R-R interval variation analysis every 2 minutes, identified AF patients with a sensitivity of 96 % and specificity of 85 %.35 Sensitivity is lower (85 %) when considering the detection of all episodes of AF.35,36 Diagnostic accuracy for AF is even higher with the new-generation Reveal LINQ (Medtronic Inc), which also has a P-wave detection algorithm (sensitivity of 97 % and specificity of 97 % for AF patient detection and sensitivity of 97 % for detection of all episodes of AF ≥2 minutes in duration).36 Both the Reveal XT and the Reveal LINQ data on AF burden are highly correlated with Holter recordings (Pearson coefficient >0.9).35,36
In a CareLink Network remote monitoring cohort study of ILR performance in detecting all types of tachyarrhythmias using a R-R interval-based algorithm, 64 % of episodes detected were true tachycardia.42 Common artefacts included signal noise, T-wave and P-wave oversensing. VT and fibrillation that can be induced with programmed electrical stimulation are detected by an ILR with a sensitivity of 99 %.42
Risk Stratification in Patients at Risk of Sudden Cardiac Death
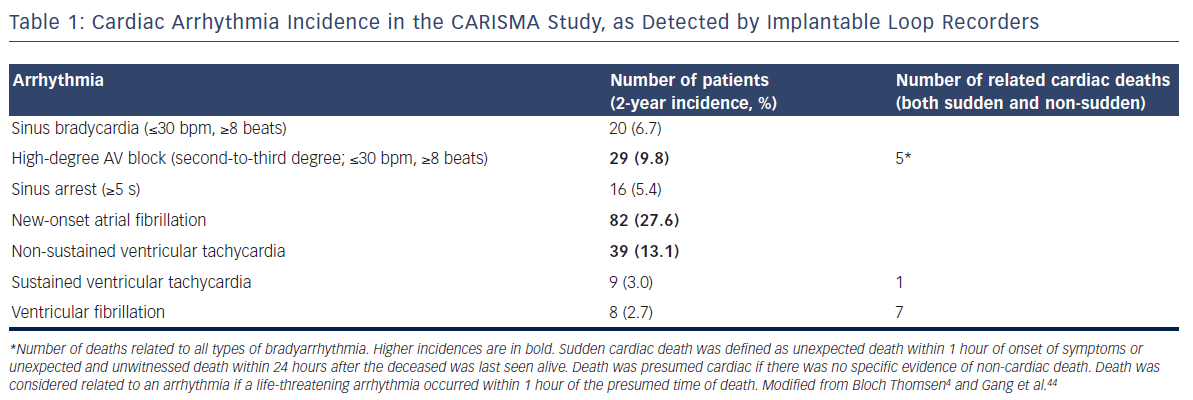
An active field of research is the use of loop recorders for the prognostic stratification of patients at increased risk of sudden cardiac death. The Cardiac Arrhythmias and Risk Stratification After Myocardial Infarction (CARISMA) study recruited 312 patients who had a left ventricular ejection fraction ≤40 % in the acute phase (up to 21 days) of a myocardial infarction to receive an ILR (Reveal Plus 9526, Medtronic Inc).4,43 Over a period of 2 years, the ILR detected arrhythmias in 46 % of patients, with the majority of episodes being asymptomatic and occurring in the first 3 months after myocardial infarction.44Table 1 reports the incidences of different types of arrhythmias in this study. Despite optimal therapy, 27 patients (9 %) died of cardiac causes (lethal arrhythmias, myocardial infarction, heart failure) during the follow-up; 13 of these were arrhythmic deaths (Table 1). Twenty-five patients (8 %) experienced at least one fatal or near-fatal arrhythmia (symptomatic sustained VT or ventricular fibrillation) that was potentially treatable by an ICD and in 17 cases (five deaths) diagnosis was made with the ILR recordings.45 Considering that none of the patients with an ICD had a sudden death during the 2-year follow-up,45 an ILR may prompt the early implantation of an ICD when potentially life-threatening ventricular arrhythmias are present, even when the patient is asymptomatic (a sustained VT occurred asymptomatically in about 50 % of cases).4 A Cox regression analysis performed by the CARISMA study group showed that sustained VT neither predicted cardiac death nor all-cause mortality.4 However, after an episode of sustained VT or ventricular fibrillation was documented on the ILR, an ICD was implanted for secondary prevention.4 For this reason the value of sustained VT as a predictor of cardiac death (in particular sudden death) could have been underestimated. The rhythm disturbances that predicted cardiac death were sinus bradycardia (HR 4.15; 95 % CI 1.37–12.62; P<0.05) and high-degree atrioventricular block (HR 6.75; 95 % CI 2.57–17.84; P<0.001).4 Mortality was evenly distributed among patients with high-degree atrioventricular block whether or not they received a pacemaker or an ICD, probably indicating that severe bradyarrhythmias (≤30 bpm, ≥8 beats) are associated with more advanced structural heart disease and a worse prognosis despite appropriate pacing.4,43 Apart from these important conclusions, it should be noted that the ILR used in the CARISMA study had some programmability limitations. For example, tachyarrhythmias were detected only if they were ≥16 beats in duration and ≥125 bpm in heart rate; some arrhythmias, such as AF with ventricular rates <125 bpm and slow VT, could have been underdetected. New-generation devices with increased sensitivity may show a higher incidence of arrhythmias in post-myocardial infarction patients.
Ongoing Studies on the Prognostic Value of Loop Recorders
ILRs have been used in the evaluation of unexplained recurrent syncope in patients with structural heart disease, showing a higher prevalence of arrhythmic causes in this group.46,47 The benefit of ILR-guided therapy in patients at high risk of sudden cardiac death, e.g. those with a reduced left ventricular ejection fraction, should be evaluated in a large clinical trial randomising patients to receive an ILR or no ILR. Data from the US Ventricular Tachyarrhythmia Detection by Implantable Loop Recording in Patients with Heart Failure and Preserved Ejection Fraction (VIP-HF) prospective observational study (NCT01989299) will extend our knowledge on the burden of arrhythmias amenable to ICD treatment in patients with structural heart disease and normal or near-normal ejection fraction. Another on-going study, the Identifying High Risk Patients Post Myocardial Infarction with Reduced Left Ventricular Function Using External Loop Recorders (INSPIRE-ELR) trial (NCT01995552) has been designed to describe the incidence of any post-myocardial infarction arrhythmia, whether it requires a specific therapy or not. The main difference with CARISMA is that INSPIRE-ELR uses an ELR (NUVANT MCT system, Corventis Inc) instead of an ILR.
ILRs could be used in diagnosing or excluding arrhythmias in patients with inherited or acquired cardiomyopathies (Brugada syndrome, long or short QT syndrome, hypertrophic cardiomyopathy, arrhythmogenic right ventricular dysplasia, amyloid cardiomyopathy, etc.) and recurrent syncope or palpitations that remain unexplained despite extensive in-hospital evaluation.2 Data on ILR use in these rare diseases are limited and mainly retrospective48–50 and the safety of an ILR approach for distinguishing benign causes of syncope and palpitations from lifethreatening arrhythmias requiring an ICD or a pacemaker should be evaluated in clinical trials.
In the Cardiac Arrhythmias in Epilepsy (CARELINK) study (NCT01946776), an ILR will be implanted in patients with difficult-to-treat epilepsy who are at increased risk of sudden unexpected death in epilepsy. The aim of the study is the description of the 2-year incidence and prevalence of clinically relevant arrhythmias, especially those that are seizure-related. Collected data will possibly clarify the pathogenic mechanisms of sudden unexpected death in epilepsy and suggest new prevention strategies.
Holter-derived Parameters as Predictors of Sudden Cardiac Death
Continuous ECG recordings may be used to analyse the autonomic control of the heart, which may be expressed by heart rate variability and heart rate turbulence.51,52 Heart rate variability is determined from the periodic variations in R-R intervals, which are driven by the sympathetic and parasympathetic modulatory activities, while heart rate turbulence is determined by analysing the variations in R-R intervals that follow premature ventricular contractions.51,52 Imbalanced autonomic control of the heart may trigger a sudden cardiac death.52 Other measures like QRS duration, QT dispersion and the changes in beat-to-beat T-wave amplitude and duration (T-wave alternans) indicate anomalies in intra-myocardial impulse propagation or in ventricular repolarisation; both alterations may act as substrates for lethal arrhythmias.52
In the CARISMA study, Holter monitoring was performed 1 and 6 weeks after an acute myocardial infarction to test several parameters as predictors of the primary endpoint of fatal or near-fatal arrhythmia that is potentially treatable by an ICD.45 Among left ventricular ejection fraction, heart rate variability and turbulence, signal-averaged ECG, QRS duration and QT dispersion, maximum work load and heart rate on exercise, number of ventricular premature beats and nonsustained VT, T-wave alternans and programmed electrical stimulation, only heart rate variability (in particular a reduced very-low-frequency component <5.7 ms2) and induction of sustained monomorphic VT with programmed electrical stimulation effectively predicted the primary endpoint.
The measure of heart rate variability could be a non-invasive marker of cardiac autonomic dysfunction, but its clinical use is limited by poor standardisation of methods.52
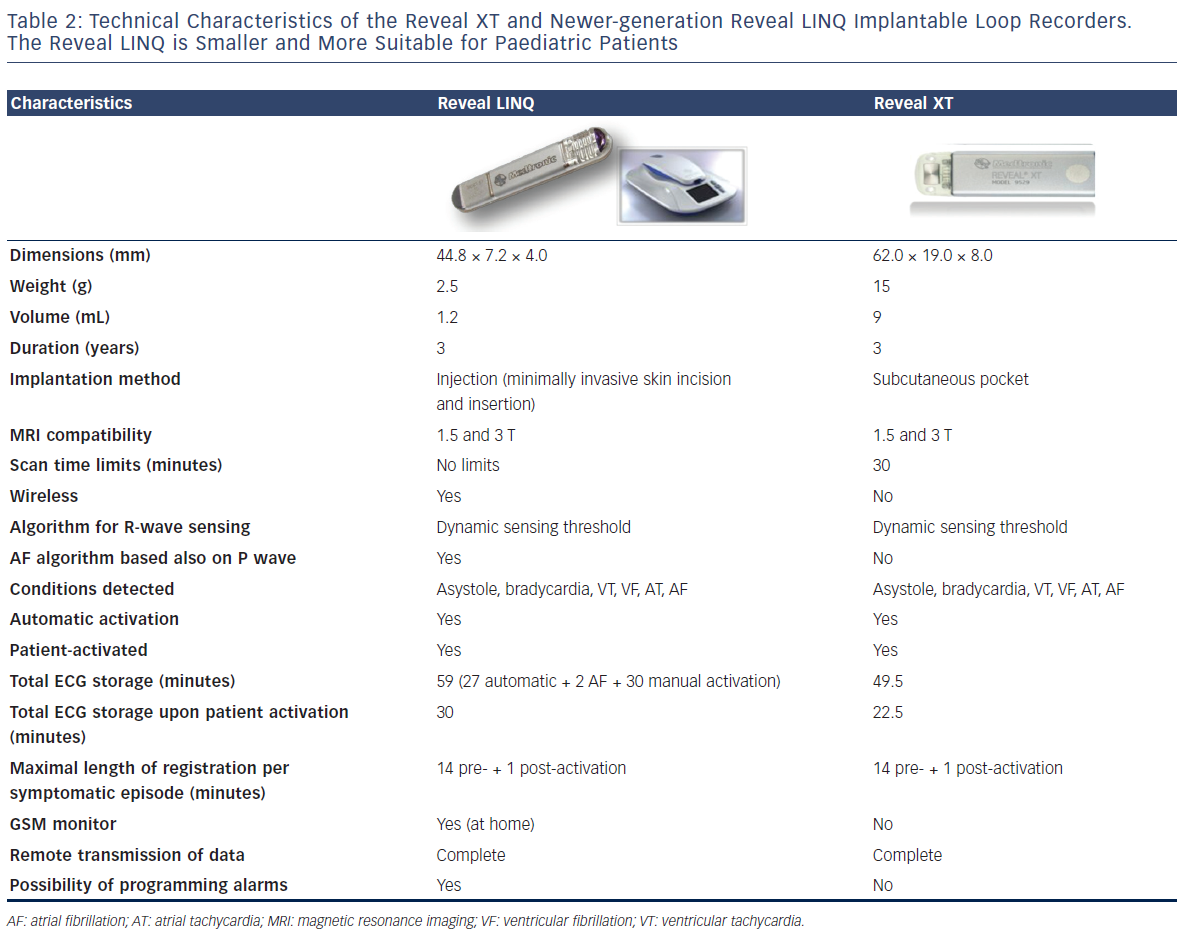
Loop Recorders in Children
The utility of ILRs has been demonstrated in several series of paediatric patients, with the majority of cases of recurrent unexplained syncope and palpitations receiving a diagnosis (between 50 and 70 %).53–59 Most frequently complaints are associated with normal sinus rhythm or neurally mediated sinus bradycardia, especially when there is no history of congenital or acquired heart disease.58–59 These findings provide reassurance of the benign nature of some symptoms. The patient, his/her family or bystanders should activate the device every time symptoms occur, because sinus rhythm is not automatically recorded. In cases where a clinically significant or lifethreatening arrhythmia is diagnosed with the ILR, a specific therapy is generally required, such as the implantation of an ICD, a pacemaker, transcatheter ablation or drug therapy.58,59
ILRs have been effectively implanted in children under 1 year of age53,54 and may have a higher diagnostic yield than ELRs, especially in younger children, as they do not have any external wearable part and do not require a strict patient collaboration. The development of new miniaturised ILRs, such as the Reveal LINQ, may increase the use of ILRs in paediatric patients. Table 2 summarises the characteristics of the Reveal LINQ as compared with those of the previous model, the Reveal XT.
Patient-activated event recorders (non-looping) are an alternative to ILRs in children who can receive a training to perform self monitoring when they have symptoms. Park and colleagues reported a high diagnostic yield with the Omron HeartScan 801 (Omron Corporation, Kyoto, Japan) event recorder, an external device that registers a single-channel ECG after it is manually activated and put on the chest.60 Of 30 patients with paroxysmal palpitations (aged 4–16 years) a clinically-significant arrhythmia could be excluded in 26 patients, who had sinus tachycardia during symptoms. Four patients received a diagnosis of supraventricular tachycardia and they were successfully ablated.
Discussion
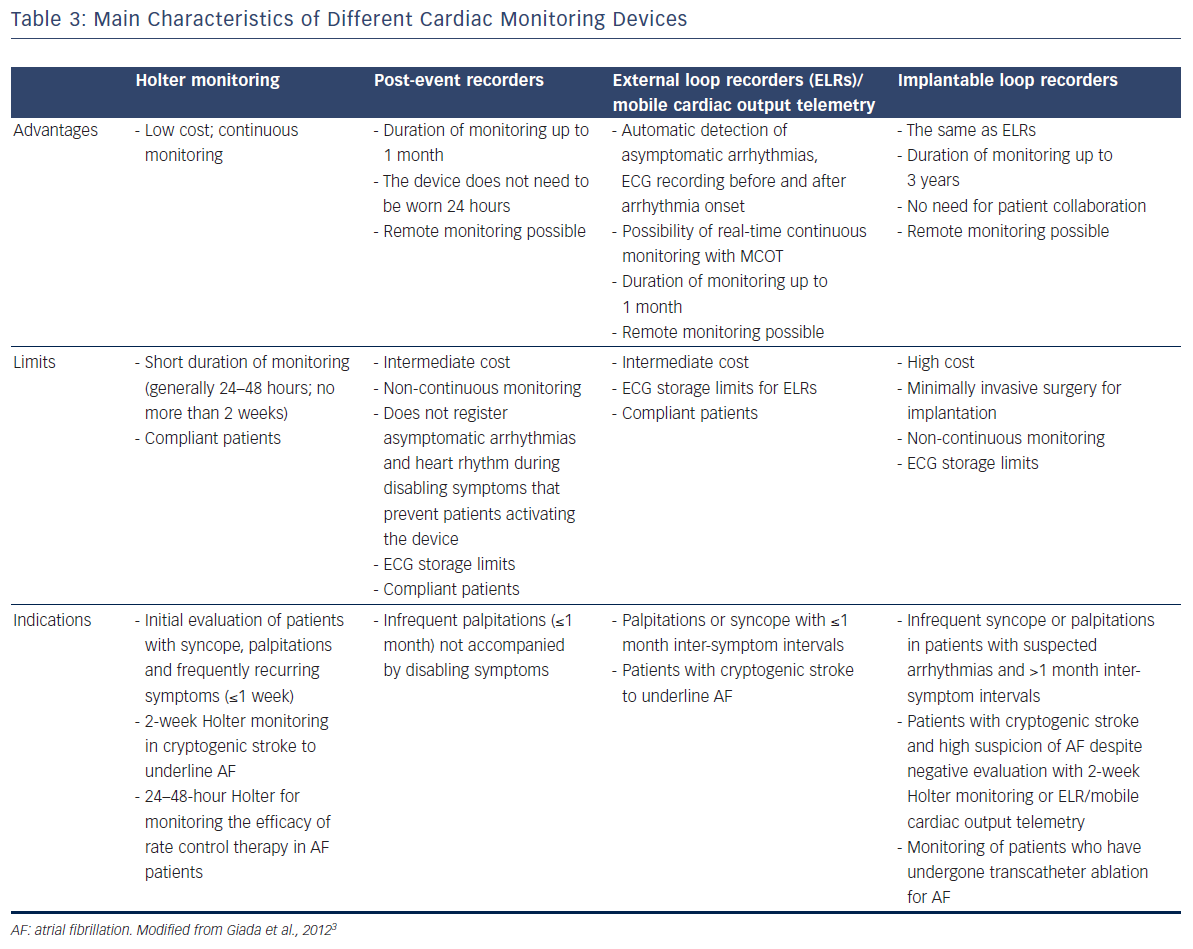
Many devices are available that allow for monitoring of heart rhythm and rate in patients with suspected or known arrhythmias (Table 3). Loop recorders and MCOT are designed for long-term monitoring of cardiac rhythm and they can provide a diagnosis in silent AF patients even when traditional Holter monitoring has failed, thus guiding therapy in patients with cryptogenic stroke.5,22,61 The prognostic utility of ILRs in patients at risk of arrhythmic death should be evaluated in large studies such as CARISMA, in the hope that in future ILR findings will help the clinician in deciding when an ICD or a pacemaker is appropriate. Remote transmission of ECG with loop recorders, MCOT, pacemakers and ICDs may facilitate the follow-up of patients, possibly reducing the economic burden on the healthcare system.1 Devices with even more sensitive algorithms for automatic detection of arrhythmias are under development.2 In future, loop recorders may provide real-time indications on haemodynamic parameters, such as blood pressure and intra-thoracic fluid status.2 Another possible direction of research is the development of ILRs sensing ST segment changes, in order to monitor therapy in patients with chronic ischaemic heart disease.2
This review focuses on loop recorders. Despite recommended use, only a minority of patients receive an ILR as diagnostic tool.62 We hope that better knowledge of the advantages, limits and indications of cardiac monitors will prompt better adherence to current guidelines.
Clinical Perspective
- This review summarises the indications, advantages and limits of different cardiac monitoring systems: Holter monitors, loop recorders, post-event recorders and mobile cardiac outpatient telemetry (MCOT).
- Loop recorders are useful diagnostic tools to underline paroxysmal atrial fibrillation in cryptogenic stroke. In future, these devices may also play a role in establishing a prognosis and guiding therapy in patients at high risk of sudden death (e.g. in patients with cardiomyopathies or heart failure).
- Loop recorders and event recorders are useful diagnostic tools in confirming or excluding arrhythmias even in children.