Idiopathic ventricular arrhythmias (VAs) are comprised of ventricular premature depolarisations, non-sustained ventricular tachycardia (VT)and rarely sustained VT, and these typically occur in the absence of structural heart disease. In general, idiopathic VAs tend to have a benign prognosis, although a high burden of VAs can result in left ventricular (LV) systolic dysfunction and cardiomyopathy and, rarely, outflow tract (OT) VAs can serve as a trigger for idiopathic VF. The mechanism of these arrhythmias tends to be triggered activity or abnormal automaticity and this usually manifests as a focal source. The 12-lead ECG morphology of the VA is helpful in predicting the site of origin (SOO). Both activation and pace mapping are utilised in localising the SOO and successfully targeting it during catheter ablation.
Idiopathic VAs tend to originate most commonly from the ventricular outflow tract (OT) region, but they can also arise from the His-Purkinje system, the papillary muscles, and other perivalvular locations. OT VAs can originate from the right and left ventricular outflow tracts (RVOT or LVOT), either above or below the pulmonic and aortic valves. They can also originate in the vicinity of the great cardiac vein and/or the anterior inter-ventricular vein, and the LV summit region. This review summarises electrocardiographic localisation of different OT VAs, our general approach to mapping and ablation of these VAs, and strategies for managing some challenging scenarios.
ECG Localisation of Outflow Tract Ventricular Arrhythmias
Differentiating Site of Origin
Analysing the 12-lead electrocardiogram morphology of the VA is the most important aspect of pre-procedural planning.1 Table 1 summarises characteristic patterns of VAs originating from different sites in the OT region. While all OT VAs will exhibit an inferior axis in the limb leads, the precordial leads are especially helpful in localising the precise SOO.
Generally, OT VAs with right bundle branch block (RBBB) morphology (predominantly positive forces in lead V1) originate from the LVOT. Meanwhile, those with left bundle branch block (LBBB) morphology (predominantly negative forces in lead V1) can originate from either the RVOT or septal aspect of the LVOT. The precordial QRS transition can be helpful in differentiating between the two. OT VAs with LBBB and late precordial transition (V4 or later) are more likely to be successfully ablated from the RVOT, while those with early transition (V1 or V2) usually originate from the LVOT (above or below the aortic valve), or the LV summit. Frequently, OT VAs will exhibit a V3 precordial transition, and these VAs can originate from either the RVOT or LVOT.
Calculating the V2 precordial transition ratio can help predict RVOT versus LVOT SOO for patients with OT VAs exhibiting V3 precordial transition. The V2 transition ratio is defined as [R/(R+S) during VA]/[R/(R+S) in sinus rhythm].2 A V2 transition ratio ≥0.6 predicts LVOT origin with >90% accuracy. While this methodology can accurately localise the SOO in the majority of cases, calculating the ratio can be tedious. A simpler and more practical approach is to compare the precordial transition of the VA versus the QRS transition during sinus rhythm. When the precordial QRS transition of the VA occurs later than QRS transition observed during sinus rhythm, the VA SOO is most likely to be from the RVOT and vice versa.2 Of note, the V2 transition ratio and/or the more simpler approach described above may not accurately predict VA SOO in patients with baseline conduction system disease such as underlying RBBB, LBBB, or left anterior fascicular block, as these patients were excluded or not well represented in the original study cohort.2
Another relatively simpler method to differentiate RVOT from LVOT SOO of VAs manifesting LBBB morphology and inferior axis is to calculate the V2S/V3R ratio during OT VA. Yoshida et al. showed that a V2S/V3R cutoff value of ≤1.5 predicted LVOT origin with 89% sensitivity and 94% specificity.3 Furthermore, for PVCs with LBBB and rightward inferior axis (with R<S in leads I and V1), measuring the R wave amplitude in lead I can differentiate RVOT from LVOT SOO. For these patients, Xie et al. showed that the presence of R wave amplitude ≥0.1 mV in lead I predicted site of successful ablation from the aortic sinuses of Valsalva or LV endocardium with 75% sensitivity and 98.2% specificity.4
Right Ventricular Outflow Tract
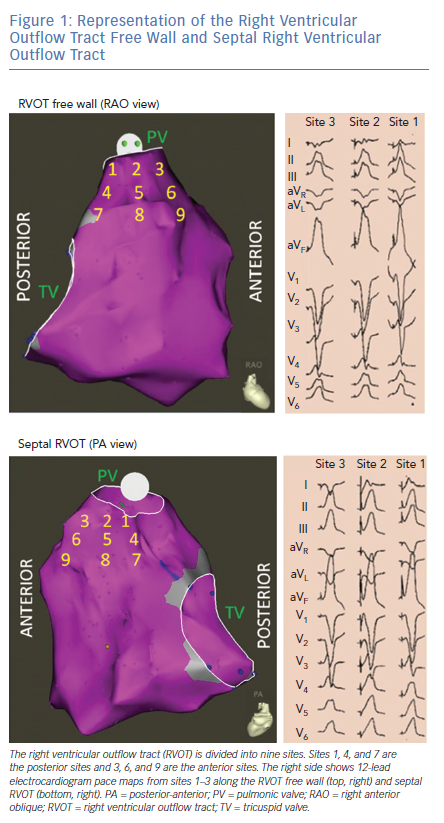
OT VAs can originate from the septal or free wall aspect of the RVOT beneath the pulmonic valve, or from above the pulmonic valve. Our group has previously reported typical ECG findings of OT VAs from different locations within the RVOT based on pace mapping in structurally normal hearts.5–7 Septal versus free wall RVOT site of origin can be distinguished based on QRS morphology. Leads II, III and aVF tend to be taller and narrower for septal compared versus free wall sites within the RVOT. Meanwhile, VAs originating from the free wall of the RVOT tend to manifest later precordial transition (≥V4) and more frequently exhibit notching of the QRS in the inferior leads.5
The septal and free wall aspects of the subpulmonic RVOT can be further segmented into nine different anatomic sites (Figure 1).7 Sites 1–3 represent the most superior sites just beneath the pulmonic valve, with site 1 being most posterior and site 3 being most anterior. Sites 4–6 are just inferior to sites 1–3, while sites 7–9 are the most inferior sites and within close proximity to the bundle of His region (RV inflow tract). Lead I can be helpful in differentiating between the posterior (more rightward, positive QRS morphology in lead I) and anterior (more leftward, negative QRS morphology in lead I) sites in the superior RVOT (sites 1,2 and 3 in Figure 1).
RVOT VAs are increasingly being recognised to originate from supravalvular myocardial extensions above the pulmonic valve.8–12 These VAs manifest similar ECG features as those arising from the superior RVOT (beneath the pulmonic valve), although they tend to be more likely to have earlier QRS transition (V2 or V3), aVL/aVR Q wave ratio >1, Qs or rS pattern in lead I, and larger V2 R/S amplitude.12
Basal Left Ventricle, Left Ventricular Outflow Tract and Aortic Sinuses of Valsalva
Our more contemporary experience shows that OT VAs are increasingly targeted from the basal LV, and the LVOT both above and below the aortic valve. The superior interventricular septum is comprised of the anterior aspect of the LVOT and the posterior aspect of the RVOT. The aortic valve sits slightly inferior to the pulmonic valve and is tilted rightward such that the anterior-most sinus of Valsalva (the right sinus of Valsalva; RSV) sits adjacent to the posterior septal aspect of the superior RVOT, while the commissure between the RSV and left sinus of Valsalva (LSV) are closer to the anterior septal aspect of the superior RVOT and the pulmonic valve. Supravalvular muscle sleeves extending above the LSV and RSV are common sites of OT VA origin.13 Furthermore, due to the anatomic proximity of the RSV and LSV to the LV summit region, these locations can be used as a vantage point for targeting OT VAs originating from the LV summit.
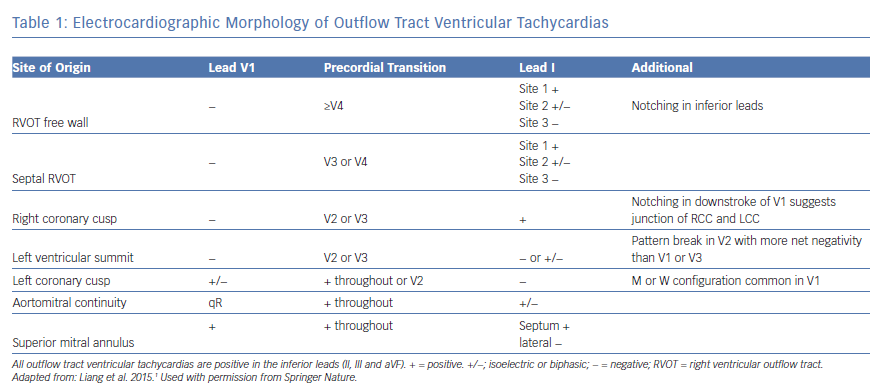
VAs from the basal LV endocardium manifest a RBBB morphology with inferior axis, except for VAs from the high left interventricular septum which may manifest LBBB morphology with early precordial transition (≤V2). We have previously demonstrated distinct ECG features with pace mapping from the different sites such as aortomitral continuity (AMC), and superior, lateral, and superolateral mitral annular regions within the basal LV endocardium in patients with structurally normal hearts (Figure 2).14 Pace maps from the AMC region typically manifest a unique qR pattern in V1 with a predominantly positive QRS complex in lead I, while mitral annular sites typically will have monophasic R waves in V1 with either positive precordial concordance or very late S wave (≥V5).14
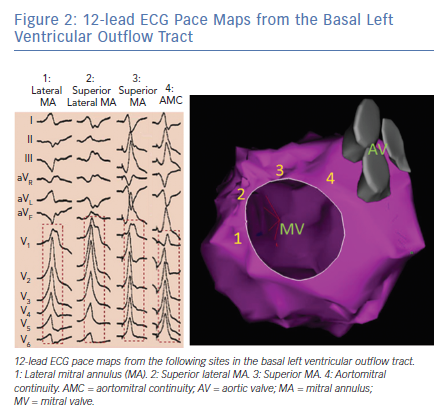
Pace mapping studies have clarified the typical QRS pattern of OT VAs originating from the aortic sinuses of Valsalva (Figure 3).15 OT VAs from the LSV manifest early precordial transition (V1 or V2) with multiphasic QRS complex with M or W patterns in V1. Meanwhile, those from the RSV tend to have LBBB with slightly later transition (V2 or V3). OT VAs from the RSV/LSV junction frequently exhibit a unique ECG pattern with a QS morphology in lead V1 with prominent notching of the downward deflection.16
Importantly, as patients age, the orientation between the LV and aortic root can alter and this can influence the QRS morphologies of OT VAs originating from the aortic sinuses of Valsalva. Specifically, older patients tend to manifest less positive forces in the inferior leads for VAs originating in the RSV because this region tends to shift more inferiorly with age.17
Left Ventricular Summit
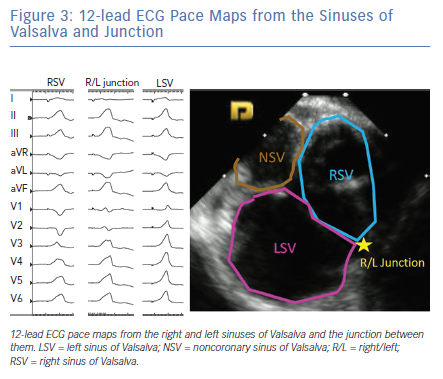
The LV summit refers to the triangular region of myocardium located at the most superior, septal and epicardial aspect of the LV (Figure 4). Idiopathic VAs are increasingly being recognised to originate from this location and this site is typically in close anatomic proximity to the epicardial coronary arteries which can be a challenge for ablating these arrhythmias. VAs originating from the LV summit typically manifest either RBBB pattern or LBBB pattern with early (V2 or V3) transition and inferior axis. LV summit VAs usually have Q wave in lead I, with lead III to lead II ratio >1.25 and QS amplitude ratio in leads aVL and aVR >1.75.18 LV summit arrhythmias can be targeted from a number of vantage points:
- LV endocardium beneath the aortic valve;
- aortic sinus of Valsalva;
- septal RVOT;
- coronary venous system (great cardiac vein and/or anterior inter-ventricular vein); and
- direct epicardial approach.
The latter can be performed in the EP laboratory (using the Sosa technique for epicardial access) or surgically. In rare instances, ablation of LV summit VAs has also been accomplished from the left atrial appendage.19 ECG characteristics can be helpful to predict the success of an epicardial ablation approach for LV summit VAs. VAs with larger maximal deflection index (≥0.55) and longer intrinsicoid deflect time (interval to peak R in V2) (>85 ms) are likely to have epicardial origin.
Hayashi et al. described that the presence of a V2 pattern break (defined as abrupt loss of R wave amplitude in V2 versus V1 and V3) suggests SOO adjacent to the anterior interventricular groove, likely within close proximity to the left anterior descending coronary artery.20 Lin et al. proposed using the aVL/aVR Q wave ratio for predicting the successful ablation site for LV summit VAs.21 In their study, an aVL/aVR ratio ≤1.415 was associated with successful ablation from the aortic sinuses of Valsalva, a ratio of 1.416–1.535 for successful ablation from the subvalvular LV, a ratio of 1.536–1.740 for successful ablation from the great cardiac vein/anterior inter-ventricular vein, while a ratio of >1.74 required direct epicardial ablation approach. In our experience, ECG predictors of successful ablation of LV summit VAs via a direct epicardial approach include aVL/aVR Q wave ratio >1.85, a V1 R/S ratio >2, and absence of Q waves in V1.22
Pearls and Pitfalls for Mapping and Ablation
Since the underlying mechanism of idiopathic OT VAs is most frequently triggered activity and the source is focal, activation mapping is the most effective method of identifying the SOO. Typically, with good catheter–tissue contact, the local bipolar electrogram at the SOO should have a sharp component which precedes the QRS onset by ≥20 ms and the unipolar electrogram at this location should manifest a QS morphology with a sharp downward slope. The ideal approach is to perform dense sampling (5–10 locations) at and around the site manifesting early activation to localise the precise ablation target which should manifest on the electroanatomic map as a small (1–2 mm) focal red area. Importantly, when such mapping results in a diffuse area (≥3 mm) of early activity, the true SOO is likely to be from another location (adjacent chamber), and further detailed mapping should be performed at other locations prior to ablation. To accomplish successful ablation, multiple locations in more than one chamber may need to be mapped to determine the ideal target.
The presence of frequent VAs at the time of the ablation procedure is an important predictor of success, as it allows for detailed activation mapping. Unfortunately, some patients, despite having high-burden VAs clinically, may have infrequent or no ventricular premature depolarisations at the time of the ablation procedure. To prevent this scenario, all beta-blockers, calcium channel blockers, and antiarrhythmic medications should be withdrawn, ideally for at least five half-lives prior to the procedure date. If amiodarone is being used to achieve arrhythmia control, then our practice is to hold this drug for at least 2 weeks in advance. Ablation should be performed with minimal anaesthesia if possible, as sedation frequently can suppress these VAs. We usually avoid using propofol and benzodiazepines for sedation during these procedure and instead use remifentanyl due to its ultra-short half-life.23 Frequently, with administration of even minimal sedation at the beginning of the case (i.e. for urinary catheter insertion or venous/arterial access), VAs may disappear completely. It is therefore vital that at least some examples of the clinical VA are captured on the recording system at the beginning of the case. Having this allows for pace mapping, which can be helpful to identify the SOO. We have found that automatic pace mapping algorithms, such as PASO (CARTO, Biosense Webster) and AutoMap Score Threshold (EnSite Precision™) can be helpful to facilitate pace mapping. However, in our experience, the pace mapping algorithms utilised by different electro-anatomic mapping platforms are not completely reliable because they can show a high level of similarity (>90%) between the pace maps and the VA over a large area (5–10 mm).
Stimulation protocols are often required for patients with rare or absent clinical VA on the day of the procedure. The choice of the stimulation protocol may be determined according to the patient’s medical history, which may provide insights on the VA triggers. The most common triggers for these VAs are anxiety, exercise and caffeine, which imply catecholaminergic mediation and this is mimicked during the procedure by decrements in overdrive pacing (from the atria or ventricles) and/or by infusing isoproterenol, epinephrine, aminophylline, etc. Less commonly phenylephrine and calcium chloride can be effective in inducing VAs in the laboratory. Recognising diurnal variations in the occurrence of VAs can help guide the timing of the procedure. In addition, awareness of the strong influence that hormonal changes can have on OT VAs in women can be helpful in procedure planning.24
In rare cases, unusual stressors may need to be explored. For example, we have encountered occasional patients whose OT VAs would only manifest when they were asked to think about stressors (spouse, recent motor vehicle accident, etc). In some patients, sleep deprivation was a trigger and we asked them to stay up the night before the procedure and avoided any sedation during the ablation procedure. One patient who underwent ablation at our centre had a clinical history of VAs triggered with red wine and required consumption of red wine in the EP laboratory to bring out the clinical arrhythmia, which was then successfully targeted.
For patients with rare or absent VAs despite the above interventions, pace mapping can be a helpful approach in identifying the ablation target. Pace mapping should be performed at or slightly above threshold output with cycle length which mimics the rate or coupling interval of the clinical VA. Ideally the pace map should be a perfect match (in all 12 ECG leads) of the VA. It is important to recognise that overall, pace mapping is inferior to activation mapping in identifying the ablation target for OT VAs.25
It is important that ablation guided by a pace mapping approach should be delivered to the site(s) that are perfect matches.26 Recent advances in electroanatomic mapping systems, including the PASO and Score Map modules, which automatically compare the morphology of the paced versus the clinical QRS morphology and calculate percentage match, can provide a quantitative assessment of pace map match. However, these algorithms are prone to overlooking subtle variations, such as the presence or absence of notches in different ECG leads between the VA and the pace map. Thus ideal pace mapping requires simultaneous comparison of the paced QRS complexes with clinical VA on the electroanatomic mapping system as well as the EP recording system. In our opinion, the latter is a better platform for a qualitative comparison, but it requires a trained electrophysiologist to perform the analysis. It is also important to recognise that the accuracy of pace mapping for accurately predicting the SOO of the clinical VA may be chamber dependent. For example, while pace mapping in the RVOT can accurately replicate the clinical VA, the same is not true when pace mapping from the aortic sinuses of Valsalva. In the latter case pacing at high outputs may be necessary to capture ventricular tissue and those pace maps may not correlate perfectly with the clinical VA originating from this region. Also, clinical VAs originating in the cusp region may manifest multiple exits or ‘preferential conduction’ and the pace maps are not always able to mimic this. Yamada et al. have reported that in 25% of patients with ASV VAs, pace maps from the RVOT were actually a closer match than from the ASV region.25
General Approach to Mapping and Ablation
Intracardiac echocardiography is being increasingly used to guide ablation of OT VAs. In our experience the CARTOSOUNDTM module (CARTO, Biosense Webster) can be especially helpful since it allows for rapid creation of the anatomic shell of the LVOT, RVOT, aortic sinuses of Valsalva and coronary arteries. Once the anatomy of the entire region is created, the detailed electroanatomic mapping information acquired by catheter manipulation can be superimposed. For OT VAs with LBBB morphology and late precordial transition (≥ V4), catheter mapping in the RVOT region is typically performed first. A steerable sheath (i.e. Agilis sheath [Abbott]) can be helpful for mapping in the RVOT region. However, for OT VA manifesting LBBB morphology but with an early transition (≤V3), we will usually map the LVOT first or when an early site is not identified in the RVOT region. Our preferred approach for mapping LVOT VAs is the retrograde aortic approach (via femoral arterial access).
Sheaths can be helpful to maintain catheter stability when accessing the LV via retrograde aortic approach. While we usually use a 23 cm or 45 cm sheath advanced to the descending aorta, in cases with aortic dilatation, aortic calcification or calcification of the aortic valve in patients in whom we wish to avoid repeated crossing of the aortic valve, a long sheath such as an 81 cm SL-1 (Abbott) or a 102 cm (82 cm usable length) Agilis sheath, advanced over an ablation catheter across the aortic valve, can be helpful. In cases where we suspect the focus to be deep intramural or at an inaccessible epicardial location (LV summit region), alternative strategies can be attempted, including using half normal saline as the catheter irrigant, simultaneous unipolar or bipolar ablation, coronary venous ethanol ablation, or ablation with an irrigated needle tip catheter.27–31
Challenging Situations
QRS Morphology Shift During Ablation
The myocardial network in the OT region is extremely complex, and the wavefront from the VA origin site can spread based on myofibre orientation, resulting in variable exit sites.26 As such, on some occasions, ablation at the site of earliest activation may result in change in QRS morphology and intracardiac activation pattern (often with initial transient suppression but late recurrence with different QRS morphology during the waiting period). In our experience, this phenomenon occurs in 4% of cases.32 In the majority (65%) of these cases, repeat mapping identified a new earliest site in the adjacent chamber within close proximity to the initial ablation site, while 35% of patients had the altered recurrent VA originating from the same chamber where the original VA was targeted. Regardless of the origin of the altered VA, repeat ablation of the new site was highly effective in achieving durable VA elimination.32
Ablation of Outflow Tract Ventricular Arrhythmias with Earliest Activation from the Coronary Venous System
When the earliest activation site of OT VA is recorded within the coronary venous system, ablation can be challenging and different ablation strategies should be considered. We have previously reported outcomes of ablation of VAs from the coronary venous system demonstrating that ablation delivery at the earliest activation site, although effective, is precluded in the majority (62%) of cases due to anatomic constraints (inability to advance ablation catheter and proximity to coronary arteries).33 In these scenarios we have successfully utilised the strategy of getting as close as possible to the SOO from adjacent locations such as the basal LV endocardium, cusp region and/or the septal RVOT. We recently reported the outcome of this strategy in 53 consecutive patients where the earliest activation site was localised to the coronary venous system.34 We were able to successfully target the clinical VA from adjacent locations in nearly half of these cases. We found that the anatomical distance between the coronary venous system site and the adjacent ablation site to be the only factor in predicting success. A cut-off distance >12.8 mm between the SOO and the ablation site strongly predicted failure. In those cases alternative ablation strategy such as simultaneous unipolar or bipolar ablation from two anatomically adjacent sites, half-normal saline use or retrograde coronary venous ethanol infusion may be considered.
Conclusion
Catheter ablation is a safe and effective treatment option for patients with OT VAs. The 12-lead ECG of the clinical VA is the most important piece of information for procedural preparation. Ablation success rates are highest when VA is spontaneous or inducible at the time of procedure, which allows for detailed activation mapping. While OT VAs with SOO that are intramural or adjacent to coronary arteries can be challenging to treat, different strategies can be attempted to overcome these difficulties. Use of intracardiac echocardiography has enhanced our ability to map and successfully target the vast majority of OT VAs.
Clinical Perspective
- Catheter ablation is a safe and effective treatment option for outflow tract (OT) ventricular arrhythmias (VAs).
- The 12-lead ECG is critical in accurately predicting the site of origin of OT VAs.
- Ablation success rates are highest when VA is spontaneously seen or reproducibly induced at the time of procedure, which allows for both activation and pace mapping.
- Ablation of OT VAs can be sometimes challenging due to anatomic constraints. Different strategies can be utilised to overcome these difficulties.