Since its introduction in 2012, leadless pacemaker (LP) therapy has developed as a therapeutic alternative to conventional transvenous pacemaker (PM) therapy to circumvent lead- and pocket-related complications.1-4 To date, two LPs are available for patients with a single-chamber pacing (VVI) indication: the Nanostim Leadless Cardiac Pacemaker (LCP; Abbott) and the Micra Transcatheter Pacing System (TPS; Medtronic).
The LPs have shown to meet the pre-specified safety and performance criteria in two large prospective multicentre single-arm studies.3,4 The LPs demonstrated similar pacing performance and safety results, and exhibit high implantation success rates.5 Reynolds et al. performed a post hoc analysis of patients who received Micra TPS implantation compared with patients who underwent transvenous PM therapy in a historical control cohort. Micra TPS had fewer major complications compared to patients in the historical control cohort at 6-month follow-up: 4.0 % versus 7.4 %, respectively.3 Reddy et al. demonstrated that long-term complication rates are expected to decrease by 71 % following Nanostim LCP implantation compared to conventional transvenous PM therapy.6
Despite these promising results, there is an important challenge to consider: the end-of-life (EOL) management of LP therapy. The optimal approach at the end of service of conventional transvenous PM therapy has been studied in great detail.7,8 The subcutaneous generator is readily accessible for replacement, leaving the leads in place.7 PM lead extraction can be a high-risk procedure and is associated with serious complications, including cardiac perforation and death.8 Up to three leads can be placed intracardially, without haemodynamic compromise.7 Optimal EOL strategy of LP therapy is subject to debate. The estimated battery longevity of the LP ranges between 4.7 and 15 years, depending on pacing parameters.2,3,9 Therefore, selected patients might require multiple devices over their lifespan. Once the EOL of the LP approaches, there are two options for implanting physicians to address this problem. LPs were designed so that they can be programmed in a non-functional mode. The LP can be abandoned and an additional device may be implanted adjacent to the non-functional LP. Important concerns have been raised regarding the aforementioned option. Multiple devices in the heart may compromise cardiac function, or be a source of interference. The second replacement strategy is to extract the LP and subsequently implant a new device. However, extraction may not be feasible due to encapsulation of the device, and this will probably be more prevalent with more chronic use of LP therapy. Of note, there are situations where extraction of the LP may be necessary, such as in infection or dislocation. Recently, a battery advisory was distributed by Abbott stating that 7 of 1423 (0.5 %) patients had a battery malfunction that occurred more than two years after Nanostim LCP implantation. This battery dysfunction has not been shown to affect Micra TPS.
With the battery advisory and the more chronic use of LPs, recommendations for EOL management become increasingly important. Therefore, an up-to-date review of available evidence on retrieval of LPs is highly clinically relevant. In this review we describe the safety, feasibility and histopathological examination of LP retrieval.
Leadless Pacemaker and Retrieval Systems
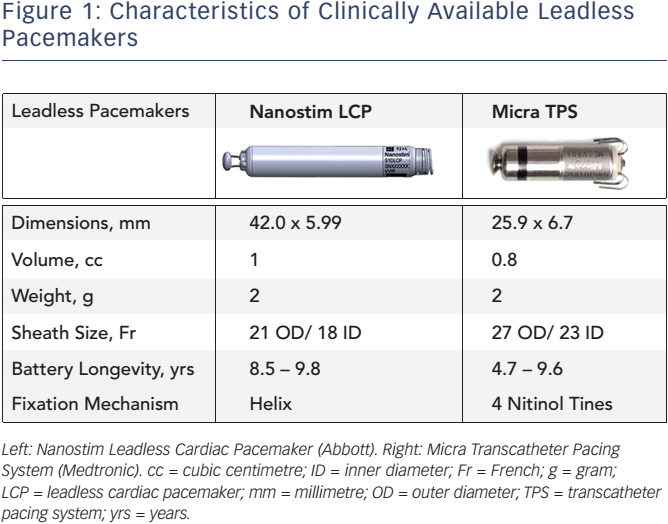
Both LPs are cylindrical intracardiac devices; however, there are differences in design that merit emphasis. The Nanostim LCP is 42 mm long and 5.99 mm in diameter, whereas the Micra TPS measures 25.9 mm in length and 6.7 mm in diameter. The characteristics of the devices are shown in Figure 1. Implantation of the devices is performed in the catheterization laboratory, under fluoroscopy. An introducer sheath (21F outer diameter [OD] for the Nanostim and 27F OD for the Micra TPS) is percutaneously placed in the femoral vein to deliver the device through the vena cava inferior towards the right ventricle (RV) using a steerable catheter. The Nanostim LCP uses an active helix to fixate the LP into the cardiac tissue of the RV. The Micra TPS is anchored into the right ventricular myocardium using an active fixation mechanism that is composed of four nitinol tines.
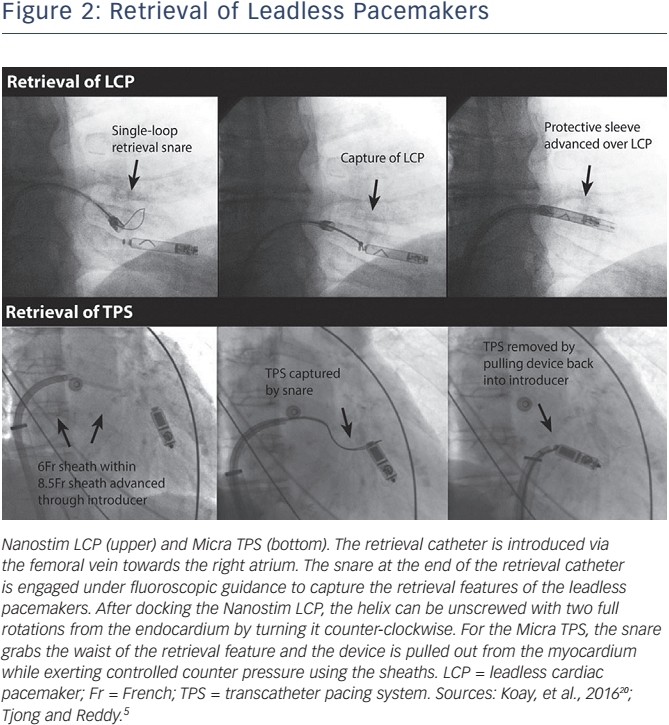
Different retrieval tools are available for the Nanostim LCP and the Micra TPS. The manufacturers of the Nanostim LCP developed a dedicated steerable retrieval catheter to allow retrieval of the device. The retrieval catheter is introduced via the femoral vein through an 18F sheath. The snare (single-loop or triple-loop) and the integrated protective sleeve at the end of the retrieval catheter are engaged under fluoroscopic guidance from the vena cava inferior towards the right atrium. The protective sleeve is retracted when positioned near the Nanostim LCP, and the single- or triple-loop snare is engaged to capture the distal cap of the LP in the RV. The snare is closed to grab the proximal docking feature of the device. After docking the Nanostim LCP, the helix can be unscrewed with two full rotations from the endocardium by turning it counter-clockwise. The protective sleeve is advanced over the total LCP, and it can be removed from the body. The Micra TPS does not have a dedicated retrieval system. It was designed with a retrieval feature at the proximal end of the LP to accommodate an off-the-shelf snare that can hold the device for removal from the myocardium. A conventional gooseneck snare alone or inserted through the delivery catheter can be used for the retrieval. The advantage of the latter option is that counter traction can be applied to the myocardium with the cup of the implant catheter. In Figure 2 the retrieval of the LP systems are illustrated.
Pre-Clinical Data on Retrieval and Multiple Implanted Leadless Pacemakers
Nanostim Leadless Pacemaker
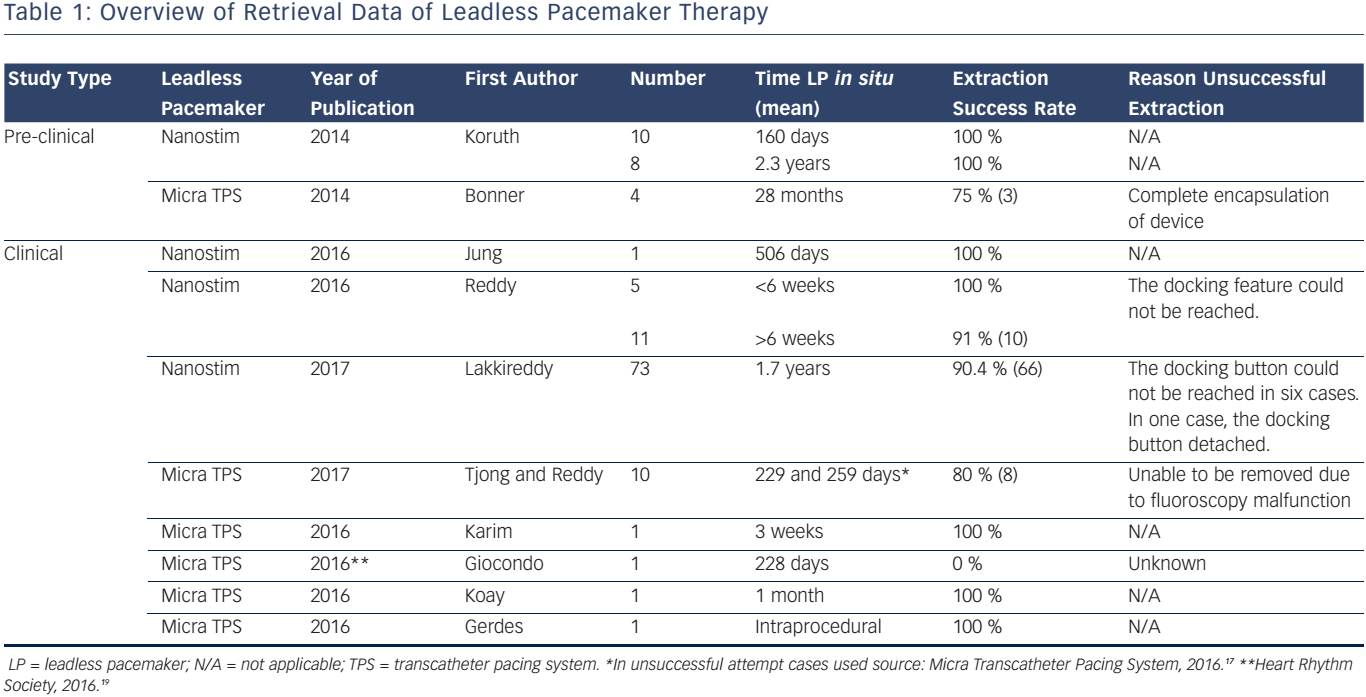
Early animal experience on retrieval of the Nanostim LCP has shown positive results. Koruth et al. evaluated the mid-term and long-term feasibility and safety of percutaneous, catheter-based retrieval of the Nanostim LCP in an ovine study.10 To evaluate mid-term retrieval capability, ten sheep underwent retrieval at a mean of 160 days, and in eight additional sheep the Nanostim LCP was extracted at a mean of 2.3 years. All mid-term and long-term retrieval attempts showed a 100 % success rate. Echocardiographic pre- and post-implant evaluation showed no signs of pericardial effusion. For the mid-term group, the time from insertion of the retrieval catheter to retrieval of the LP was 2.35 min, whereas for the long-term group this was 3.04 min. The relative short retrieval times underline the ease of the retrieval attempts. It is important to note that histological characteristics of ovine myocardium and its reaction to the device may be different compared to the human heart.
Micra Transcatheter Pacing System
Early pre-clinical animal experience demonstrated successful retrievals in three out of four ovines up to 28 months after Micra TPS implantation.11 The unsuccessful attempt was due to full encapsulation of the device. It has been suggested that a new LP can be placed adjacent to the abandoned non-functional LP. However, two important issues arise: (1) the maximum number of LPs the RV can accommodate anatomically; and (2) the effect of multiple intracardiac devices on RV function. Therefore, Omdahl et al. evaluated the number of Micra TPS that could be placed in human cadaver hearts.12 Seven hearts were successfully implanted with three Micra TPS in traditional pacing locations using standard implantation procedures. They concluded that the RV was able to accommodate three Micra TPS without physical interaction, even in a small RV of 35 cc. However, mechanical or electric interactions between intracardiac Micra TPS may be different in contracting human hearts. To assess the effect of multiple LPs on the RV cardiac function, Chen et al. sequentially implanted two Micra TPS within 1 month in 14 pigs.13 Of all pigs that underwent implantation procedures, five animals died prior to the end of the 6-month follow-up. Echocardiography was performed at baseline, at second implantation, and at the end of the 6-month follow-up. Chen and co-workers showed no significant changes in cardiac proportions based on echocardiography and no observation of injury to the tricuspid valve.
Clinical Data on Leadless Pacemaker Retrieval
Nanostim Leadless Pacemaker
Jung et al. described a case of successful retrieval of a Nanostim LCP 506 days post-implant.14 The reason for extraction was because the patient had an indication for cardiac resynchronisation therapy. Reddy et al. performed a multicentre study, wherein they evaluated feasibility and safety of retrieval before and after 6 months post-Nanostim LCP implantation in 16 patients.15 The mean time from LCP implantation to retrieval attempt was 240 days. The indications for retrieval were elevated pacing thresholds (n=8), deterioration of heart failure (n=5), pacing failure (n=1), defibrillator implantation (n=1) and elective explantation (n=1). The success-rate was 94 % (15 of 16 patients). For the unsuccessful retrieval attempt, the device had been implanted for 103 days. The docking feature could not be reached due to its location near the tricuspid valve. A new Nanostim LCP was implanted adjacent to the initial LCP, and no procedure-related adverse events were reported. In a study by Lakkireddy et al., the worldwide experience on battery failure and Nanostim retrieval was evaluated.16 An attempt for retrieval was performed in 73 patients following Nanostim LCP implantation. The time that the Nanostim LCP was implanted in the heart ranged from 0.2 to 4.0 years. In 66 of these cases the retrieval attempts were successful (i.e. 90.4 %). Another 115 patients received an additional LP or conventional transvenous PM adjacent to the abandoned Nanostim LCP due to the advisory. No adverse haemodynamic, mechanical or electrical interactions were reported. In two cases a serious adverse occurred related to the Nanostim LCP retrieval. In one case an atriovenous fistula developed and in one case the docking button detached and migrated into the pulmonary artery
Micra Transcatheter Pacing System
Tjong and Reddy reported that 13 patients who underwent Micra TPS implantation required a system revision.5 The indications for revision were due to pacemaker syndrome, elevated thresholds, upgrade to biventricular pacing, and device infection. In 8 of 10 patients the attempt for retrieval was successful. For the unsuccessful attempts, the Micra TPS were 229 and 259 days in situ.17 One of these Micra devices was snared but was unable to be removed due to fluoroscopy malfunction. In the three remaining patients who required system revision, retrieval was not attempted and the device was abandoned. Karim et al. reported the first successful extraction of a Micra TPS in a patient 3 weeks after initial device implantation.18 The Micra TPS had elevated capture thresholds. The automated capture management algorithm consequently increased the pacing output. Since the expected battery longevity would decline substantially, the physicians decided to retrieve the Micra TPS and subsequently implant a new LP. In contrast, one case was presented at the 37th Heart Rhythm Society Scientific Sessions (San Francisco, CA, USA) that demonstrated an unsuccessful retrieval of the device implanted after 228 days.19 Koay et al. was the first investigator who described the extraction of an infected Micra TPS.20 The patient developed symptoms of infection 1 month after Micra TPS implantation. Transoesophageal echocardiography demonstrated a vegetation attached to the proximal part of the device. Device interrogation demonstrated elevated capture threshold and increased pacing output. Therefore, it was decided to extract the Micra TPS and this proceeded uneventfully. Gerdes et al. described a case of Micra TPS retrieval after tether removal, while no standard correctly dimensioned snare was available.21 A steerable sheath (Agilis, St Jude Medical) was engaged into the introducer; however, incongruent proportions led to blood leakage from the introducer. After manually solving this problem, a standard 6F 20-mm snare kit (Amplatz Goose Neck) was inserted to withdraw the Micra TPS. Subsequently, a new Micra TPS was successfully implanted. An overview on retrieval data of LP therapy is displayed in Table 1.
Histopathological Examination
Occurrence of encapsulation and histopathological evaluation of the LP is highly relevant as it may influence LP retrieval management. Fibrous tissue formation might complicate the recapture of the device. Therefore, multiple pre-clinical studies and case reports have been published addressing this topic.
Nanostim Leadless Pacemaker
Koruth et al. performed pathological examination of the Nanostim LCP in an ovine study in which devices had been implanted for a mean of 2.3 years.10 They showed that there was no visible tissue on the body of the LCP. There was little fibrous tissue located at the proximal docking feature, and distal helix. Some subendocardial haemorrhage was observed at the implant site in the RV apex. In the study by Reddy et al., 16 patients with a Nanostim LCP underwent a retrieval attempt at a mean of 240 days.15 Although no pathological evaluation was performed, visual inspection showed that in 10 of 16 (63 %) patients, fibrous tissue was present on the docking knob or helix, and in 1 there was near-complete device encapsulation. Tjong et al. described a patient’s postmortem histological examination at 19 months after Nanostim LCP implant.22 The evaluation revealed partial (i.e. approximately 60 %), ongoing myofibrocellular encapsulation around the Nanostim LCP.
Micra Transcatheter Pacing System
In a swine study performed by Chen et al., nine animals reached the endpoint with a mean follow-up of 215 days.13 Necropsy and histopathological examination showed little fibrous tissue around the extracted Micra TPS, and there were no observations of tricuspid valve injury. Complete encapsulation of the Micra TPS has been observed during autopsy of a patient 1 year after Micra TPS implantation.23 This Micra TPS was adherent to the adjacent papillary muscle and immunohistochemistry revealed signs of chronic inflammation around the Micra TPS. In a pre-clinical study, one of four Micra TPS was not retrievable at 28 months following implantation.11 Necropsy analysis of the unsuccessful retrieval attempt demonstrated the device was fully encapsulated. In a case report by Koay et al., the infected Micra TPS that was successfully extracted was covered with a thin layer of fibrous tissue firmly attached to all the fixation tines.20 The histopathological examination demonstrated fibrous tissue with infiltration of neutrophils and histiocytes, confirming the existence of inflammation.
Recommendations and Perspectives
Strategies to replace LPs reaching end of service remain an unsettled concern. One option is to abandon a non-functional LP and place an additional device in the RV. The volume of the LPs (0.8–1.0 cc) occupies less than 2 % of the normal RV volume,24 consequently causing negligible hemodynamic compromise. In a study by Lakkireddy et al. no devicedevice related adverse events were reported in 115 patients in whom a new device was implanted adjacent to the abandoned Nanostim LCP.16 Electrical interaction between the functioning and non-functioning device is unlikely; however, there is currently no long-term evidence to confirm this assumption. In selected cases, one can assume that attempting extraction of a fully encapsulated LP may have higher risk than leaving the device in place. Progressive encapsulation over time might even make retrieval impossible without open-heart surgery. Although the aforementioned clinical scenarios show that there are valid arguments for this EOL strategy, we suggest that the approach of extracting the LP is more appealing to limit the amount of nonfunctioning intracardiac hardware. By extracting the non-functioning LP, the potential risk for device–device interference is mitigated, as well as unknown long-term risks associated with multiple devices in situ. In addition, the option of retrieval may result in a more accessible RV in case re-implantation of an additional device is indicated.
Several strategies should be implemented to prevent early battery depletion. It is recommended to avoid relatively high pacing thresholds as they inversely affect battery longevity of the LP. Economic programming of the LP may positively influence battery longevity, especially in non-pacemaker dependent patients. In these patients, pacemaker outputs can be programmed close to the pacing threshold. Therefore, Micra TPS has an automatic capture management to ensure pacing outputs remain at safe levels while adapting outputs to maximise battery longevity.
It is evident that incorporating a long-life self-rechargeable battery, or even no battery, would provide a major improvement in cardiac pacing therapy. A permanent PM system capable of self-recharging would circumvent disadvantages related to PM replacement, and eliminate its related risks. It was shown in a pre-clinical study that lead- and batteryless pacing was feasible using its own heart motion.25 In another pre-clinical study, a batteryless PM was developed that was powered by a solar module that converted transcutaneous light into electrical energy.26 This PM was able to provide pacing therapy continuously at a rate of 125 BPM for 1.5 months in the dark.
Retrieval of the LP remains an essential consideration for patients who are potentially eligible for leadless VVI pacing therapy. The potential inability to retrieve chronically implanted devices may limit the application of this novel technology in selected cases. Although initial studies have demonstrated promising results, early experience on retrieval feasibility and safety of LP therapy is mixed. Therefore, longterm prospective analysis is required to define the most optimal EOL strategy concerning LP therapy.
Clinical Perspective
- There are two strategies to address end-of-life management of leadless pacemakers: (1) placing an additional leadless device adjacent to the non-functioning leadless pacemaker and (2) retrieving the non-functioning leadless pacemaker and subsequently implanting a new device.
- There are clinical scenarios that have valid arguments for both aforementioned end-of-life strategies.
- We suggest that the approach of LP retrieval is more appealing to limit the amount of non-functioning intracardiac hardware, mitigate risk for device interference, and limit unknown longterm complications associated with multiple chronic implanted leadless pacemakers.