Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiovascular disorder worldwide with a prevalence of 1 in 500 in the general population.1 It can be defined as a condition that is typified by unexplained left ventricular hypertrophy (LVH) in the absence of other cardiac or non-cardiac conditions that could produce hypertrophy of similar proportions.2 The condition was the first cardiomyopathy to be causally linked with a genetic mutation, and subsequent studies to date have shown that a specific mutation affecting the sarcomere or sarcomere-associated proteins can be identified in more than half the cases.3,4 Typical pathological features of the condition include hypertrophy, fibrosis and cardiomyocyte disarray.5 Other characteristics include impaired sarcomere calcium cycling, inefficient energy usage and microvascular dysfunction.6 Echocardiography is usually diagnostic of the condition in the presence of features, such as maximal left ventricular (LV) wall thickness ≥15 millimetres (mm), asymmetric septal hypertrophy, apical HCM or systolic anterior motion of the mitral valve, and septal/posterior wall thickness ratio >1.3.7 However, HCM can be phenotypically heterogeneous and also manifest with mild LVH, concentric LVH and without some of these typical characteristics, whilst retaining the increased risk for sudden death.2,7,8 This can create difficulties in terms of differentiation from other causes of LVH such as hypertension or physiological adaptations to exercise seen in athletes. An additional challenge to diagnosis is posed by the fact that mutations in genes encoding sarcomeric proteins are not found in up to a third of patients with HCM9 and the diagnosis remains a clinical rather than a ‘genetic’ one. This has spurred investigation of other genetic mutations that can cause LVH. Several other genetic conditions that are not caused by cardiac sarcomere mutations have been associated with severe LVH and its associated consequences. These conditions are referred to as HCM phenocopies and include a variety of disorders such as glycogen storage disorders, lysosomal storage disorders, mitochondrial cytopathies, cardiac amyloidosis and disorders of fatty acid metabolism.10 These conditions differ significantly from HCM due to sarcomeric mutations in terms not only of pathogenesis of hypertrophy but also of clinical features and prognosis.
Glycogen Storage Disorders
Several important features can help to distinguish these conditions from classic HCM with sarcomeric gene defects. These include evidence of multisystemic clinical features at an early age, extreme LVH (stimulated by glycogen-filled vacuoles rather than the myocyte disarray or fibrosis that is characteristic of sarcomeric HCM), early progression to dilated cardiomyopathy and electrocardiographic abnormalities such as ventricular pre-excitation and conduction system defects (see Table 1).11 Ventricular pre-excitation in these conditions has been hypothesised to be caused by the unique mechanism of disruption of the annulus fibrosus by glycogen-filled myocytes rather than the presence of histologically distinct accessory bypass tracts.12 The salient features of the individual conditions are detailed below.
Danon Disease
This important condition was originally described by Danon et al. as a lysosomal storage disease with normal acid maltase.13 It is caused by a primary deficiency in lysosome-associated membrane protein-2 (LAMP2)14 and is transmitted via X-linked dominant inheritance, although spontaneous mutations have also been described.11 The entire coding region of the LAMP2 gene is located on Xq24, including the nine exons (1–9a) and intron-exon junctions. To date, over 60 mutations have been found to cause primary deficiencies in the LAMP2 gene. These include frameshift, splicing and large deletion mutations, as well as premature stop codons. As a result of its X-linked nature, Danon disease presents more often and more severely in males than females. Individuals with LAMP2 mutations have defects in glycogen degradation pathways, leading to insidious glycogen accumulation. Intracytoplasmic vacuoles within the cardiac muscle cells can form, containing both autophagic material and glycogen. This leads to the disorganisation of ventricular myocardium and hypertrophy.
Whilst difficulties in diagnosis probably lead to an underestimation of its prevalence, LAMP2 mutations have been found in 1–8 % of patients with suspected HCM who underwent genetic testing.11,15 Heterozygous females develop later onset as well as milder symptoms in comparison with males.14 A study involving the largest series of patients with Danon disease has described the clinical features and outcome of 82 patients in 36 families.14 Typical symptoms include a triad of proximal muscle weakness (80 %), intellectual disability (100 % of men and 50 % of women) and hypertrophic cardiomyopathy (88 %).14,16 The most common initial symptoms of Danon disease are palpitations and chest pain,14 with most patients being classified New York Heart Association Cass 1 – often they are asymptomatic with no limitations to their ordinary physical activity. In some cases, patients may experience activity intolerance as well as syncope. The disease can lead to rapid cardiac deterioration, with some patients experiencing the transition from being asymptomatic to being affected by end-stage heart failure in as little as six months. In the most severe cases sudden cardiac death can occur. Men experience events of symptom onset, diagnosis and death or transplantation some 15 years earlier than women. The average ages of these events are 12.1, 17.9 and 19.0 years old in men and 27.9, 33.7 and 34.6 years in women. Males have very poor prognosis, and in the absence of cardiac transplantation death typically occurs before 25 years of age due to heart failure or arrhythmias.14,17
Clinical suspicion of the condition should prompt testing of serum creatine kinase and liver enzyme levels, both of which are usually raised in this condition. The electrocardiograph may show Wolff-Parkinson-White (WPW)-type changes (short PR interval and pre-excitation) in up to two-thirds of men and less than a third of women,14 and this finding along with very large voltage complexes especially among male teenagers should also raise suspicion of this condition. Skeletal muscle biopsy shows intra-sarcoplasmic periodic acid-Schiff positive vacuoles.15 Whilst echocardiography usually shows severe concentric LVH, asymmetric septal hypertrophy may also have been in some patients resembling the pattern seen in sarcomeric HCM, and in late stages changes of dilated cardiomyopathy pre-dominate.18 Molecular genetic screening revealing mutation of the LAMP2 gene confirms the diagnosis. There is no specific medical therapy available for the condition. Consideration should be given to implantable cardioverter defibrillator (ICD) implantation although severe hypertrophy is associated with high defibrillation thresholds and failure to terminate ventricular fibrillation (VF); there is some evidence that subcutaneous ICDs may be preferable in this respect.19 Cardiac transplantation should be considered at an early stage.18
PRKAG2 Cardiomyopathy
This condition with autosomal dominant inheritance is due to mutations in the protein kinase, Adenosine monophosphate (AMP)-activated, gamma 2 non-catalytic subunit (PRKAG2) gene, which encodes the regulatory gamma (γ)-subunit of AMP-activated protein kinase (AMPK).9,20 It presents in adolescents and young adults with muscle weakness, and imaging typically demonstrates LVH with global hypokinesia. The cardiac hypertrophy seen in this condition is usually, but not always, associated with excess glycogen deposition,21 can be variable in severity and also asymmetric, thus mimicking the pattern seen in HCM with sarcomeric gene defects. However, an important distinction from sarcomeric HCM and similar to other glycogen storage disorders, is the early progression to systolic dysfunction and dilated cardiomyopathy.11 This condition can also be associated with WPW syndrome and conduction system degeneration. Treatment options include pacemaker implantation for conduction disease or cardiac transplantation for heart failure; however, transgenic modulation of intracardiac glycogen levels has also shown promising results in reversal of both cardiac dysfunction as well the electrophysiological abnormalities.22
Pompe Disease (Glycogen Storage Disease Type 2)
This is an autosomal recessive disorder due to acid maltase (acid alpha [α]-glucosidase) deficiency leading to multisystemic glycogen deposition and can manifest in infantile, juvenile and adult forms. The infantile form is one of the foremost causes of HCM in the paediatric population and presents with hypotonia, macroglossia, hepatomegaly, extreme LVH and heart failure, usually leading to death within two years.16 Whilst cardiac involvement may present as dilated cardiomyopathy in a proportion of patients, LVH in the infantile form may be asymmetric and associated with LV outflow tract obstruction.23 Later onset forms have milder cardiac involvement and present with proximal myopathy. Skeletal muscle biopsy shows vacuolar deposition of glycogen and enzyme deficiency can be demonstrated in fibroblasts, lymphocytes and urine.23 Electrocardiography (ECG) shows features of LVH and may also show short PR interval with pre-excitation or conduction block. Encouraging results in terms of improvement in LVH and survival have been demonstrated in recent studies using recombinant acid α-glucosidase in patients with infantile Pompe’s disease.24
Forbes Disease (Glycogen Storage Disease Type 3)
This is an autosomal recessive inborn error of metabolism that is caused by mutations of the glycogen debranching enzyme (amylo-alpha-1, 6-glucosidase [AGL]) gene and can present among infants, adolescents or young adults.10 Typical features include muscle weakness, poor growth, hypoglycaemia and concentric LVH with a tendency to develop dilated cardiomyopathy in later years.10 Treatment includes administration of glucagon, cardiac transplantation can be considered in presence of heart failure.
Lysosomal Storage Disorders
Anderson-Fabry Disease
This condition is caused by a mutation in the α-galactosidase A (α-GAL A) gene and leads to multisystemic lysosomal accumulation of glycosphingolipids.16 The α-galactosidase A gene is localised to the long arm of the X chromosome (locus Xq22.1). As Fabry disease is an X-linked condition, heterozygous mothers have a 50 % chance of passing the defective gene on to all offspring. Sons who inherit the gene will have Fabry disease. Daughters, once thought to be asymptomatic carriers, may in fact develop disease manifestations from mild to severe25 because of X-inactivation.
Symptoms usually manifest in childhood (typically before 10 years of age). These include cutaneous angiokeratoma, hypohidrosis, acroparaesthesiae, gastrointestinal disturbances and corneal dystrophy.25 Despite these, diagnosis can be delayed by approximately 15 years.25 Life-threatening multi-organ involvement such as renal (proteinuria, end-stage renal failure), cardiac (angina due to microvascular ischaemia, arrhythmias, conduction disease, systolic and diastolic dysfunction) and cerebrovascular complications (strokes) occured in the third and fourth decade. LVH diagnosed by echocardiography has been described in about half of male patients with Fabry’s disease with age of onset of 38 years, and is due to abnormal accumulation of glycosphingolipids.25 The pattern of hypertrophy is concentric in the majority of these patients,16 which may help distinguish from the asymmetric hypertrophy seen in sarcomeric HCM. A ‘cardiac variant’ of Fabry’s disease has also been described among six out of 153 patients referred for evaluation of HCM, only one of whom had symptoms of Fabry’s disease.26 Patients with the cardiac variant have residual activity of the α-galactosidase A enzyme activity and tend to present in middle age.16,26
Investigations reveal reduced α-galactosidase A activity. ECG is abnormal in most cases with voltage criteria for LVH. Short PR interval and conduction disease may also be found.16 Echocardiography shows concentric LVH, diastolic and in later stages systolic dysfunction. Cardiovascular magnetic resonance (CMR) also shows concentric LVH with characteristic late gadolinium enhancement (LGE) of the basal inferolateral wall.27
There is no cure for Fabry disease. The treatment of patients with Fabry disease primarily focuses on replacing the missing or deficient enzyme (α-galactosidase A, or α-Gal A). All classically affected males (i.e. with very low or undetectable levels of α-Gal A) should receive enzyme replacement therapy (ERT) as soon as the diagnosis is made, regardless of whether or not clinical manifestations are present. Female carriers and atypically affected males (i.e. with marginal levels of -Gal A) should receive ERT if clinical manifestations (e.g. renal, neurologic, cardiovascular) are present. The impact of enzyme replacement therapy on mortality is unknown.
Cardiac Amyloidosis
Amyloid cardiomyopathy is due to extra-cellular deposits of amyloid in the myocardium and can complicate both primary Amyloid Light-chain (AL) amyloidosis (in about 50 % patients) as well as transthyretin amyloidosis.28 Cardiac involvement significantly worsens prognosis in amyloidosis and symptoms include chest pain, symptoms of heart failure, arrhythmias and sudden death.28 The condition is also characterised by multisystem involvement such as carpal tunnel syndrome, easy bruising, macroglossia, neuropathy and hepatomegaly. The ECG characteristically shows low-voltage QRS complexes. Echocardiography shows bi-ventricular hypertrophy with valve thickening, bi-atrial dilatation and diastolic dysfunction.29 Special echocardiographic techniques such as strain and strain rate imaging, derived from speckle tracking, can help to distinguish from sarcomeric HCM.29 Whilst the pattern of LVH is usually concentric and non-obstructive in cardiac amyloid, asymmetric obstructive forms have also been described, thus mimicking sarcomeric forms of HCM.30 CMR showing global, subendocardial late gadolinium enhancement is pathognomonic of cardiac amyloid and also predicts prognosis of the condition.31 Treatment of cardiac amyloidosis is generally supportive due to the poor prognosis of the condition. Both standard heart failure medication and device therapy produce limited benefit.29 Cardiac transplantation can be useful in selected patients. Another approach is to reduce amyloid fibril precursor protein production using combination therapy using cyclophosphamide, thalidomide and dexamethasone, novel therapies such as proteasome inhibitors (bortezomib) or the newer immunomodulatory drugs (lenalidomide and pomalidomide).29
Mitochondrial Cytopathies
This is a heterogeneous group of disorders caused by mutations of the maternally inherited mitochondrial genome and leads to dysfunctional energy production and multisystemic involvement (particularly central nervous system, heart and skeletal system).32 Affected individuals can present with symptoms any time from infancy to adulthood. Typically non-obstructive cardiomyopathy with mild concentric hypertrophy is found in about a quarter of patients and this significantly worsens prognosis with around a half of these patients deteriorating to develop heart failure and about 70 % mortality before 30 years.32 Cardiac transplantation can be considered in patients with heart failure.
Other Inborn Errors of Metabolism
Hypertrophic patterns of cardiomyopathy can also complicate other inborn errors of metabolism such as mucopolysaccharidosis (particularly Hurler syndrome), sphingolipidosis (such as Gaucher’s and Niemann Pick’s disease) and certain types of fatty acid metabolism defects, and worsen prognosis due to these conditions.
RASopathies (Malformation Syndromes)
The two most common malformation syndromes associated with HCM are Noonan syndrome and LEOPARD syndrome (Lentigines, Electrocardiographic abnormalities, Ocular hypertelorism, Pulmonic stenosis, Abnormal male genitalia, Retardation of growth and Deafness), which is the allelic variant of Noonan syndrome. These are examples of RASopathies or developmental syndromes caused by germline mutations in genes that alter the Ras subfamily. Noonan syndrome has an autosomal dominant mode of inheritance and hypertrophic cardiomyopathy can be found in up to a quarter of these patients.32 The majority of Noonan patients also have other congenital heart diseases such as aortic and pulmonary valve stenoses and atrioventricular septal defects.
Hypertensive Heart Disease
Left ventricular hypertrophy secondary to hypertension can be difficult to distinguish from non-obstructive HCM caused by sarcomeric mutations as there can be an overlap (up to 25 %) in the patterns of hypertrophy seen in both conditions.33 Similarly, other features of HCM such as systolic anterior motion of the mitral valve (SAM) or dynamic left ventricular outflow tract (LVOT) obstruction due to basal septal hypertrophy leading to dynamic LVOT obstruction, can also be seen in a proportion of patients with LVH secondary to hypertension especially if hypertension is severe and untreated.8,34 However, SAM in such cases occurs at the end of systole compared with earlier in HCM.35 Several specialised echocardiographic techniques have been used in the differential diagnosis of HCM. Tissue Doppler imaging can help distinguish between the two, showing more impairment of diastolic function in HCM and lower early diastolic velocities.8 Two-dimensional (2D) strain echocardiography can also help to aid diagnosis as radial strain in the mid and apical short axis segments have been shown to be reduced in HCM with sarcomeric mutations.36 Similarly, systolic longitudinal strain has been shown to have value in distinguishing between biopsy-proven HCM versus hypertensive LVH, with HCM being characterised by a marked reduction.34 CMR imaging can be helping in terms of identifying fibrosis associated with HCM. Serum markers such as norepinephrine, atrial natriuretic peptide and brain natriuretic peptide tend to be higher in HCM than in hypertensive LVH patients.37 Detailed clinical assessment of relatives may be crucial in making a diagnosis, as the identification of HCM in family members dramatically increases the likelihood that LVH has a genetic basis.
Athlete’s Heart
Hypertrophic cardiomyopathy is the most common cause of sudden death among athletes.38 Systematic and endurance training can also lead to physiologic LVH and the degree of LVH can overlap with that seen in milder forms of HCM.38 Thus distinguishing this from non-obstructive HCM poses an important diagnostic dilemma and can also have far-reaching implications upon participation in competitive sports. Detailed history taking can help elicit a positive family history of sudden cardiac death, which may point towards a genetic cardiomyopathy. The standard 12-lead ECG can be very useful in identifying HCM, with ST depression, T wave inversion, abnormal Q waves or axis and atrial enlargement all increasing the likelihood of HCM. The LVH seen in female athletes does not usually reach dimensions seen in the ‘overlap zone’ with HCM (13–15 mm LV wall thickness), hence these dimensions in female athletes strongly suggest pathological hypertrophy.38 Black athletes exhibit a greater overlap with HCM, with some healthy black male athletes demonstrating LV wall thickness up to 16 mm and females up to 13 mm.39 Echocardiographic features such as outflow tract obstruction, systolic anterior motion of the mitral valve with systolic mitral annular velocity <9 centimetres per second (cm/s) and Doppler evidence of abnormal filling patterns with impaired diastolic function are not usually seen in the athlete’s heart.7 On the contrary, enlarged LV cavity dimension and a reduction in LV wall thickness by periods of de-conditioning both favour physiological hypertrophy.2 Further investigations that may be of benefit include exercise testing (to identify arrhythmias or attenuated blood pressure response), ambulatory monitoring (for arrhythmias) or magnetic resonance imaging (for evidence of fibrosis). Thus a combination of these criteria along with results of detailed family assessment can be useful to distinguish the physiological hypertrophy seen in athlete’s heart from the pathological hypertrophy seen in HCM.
Conclusions
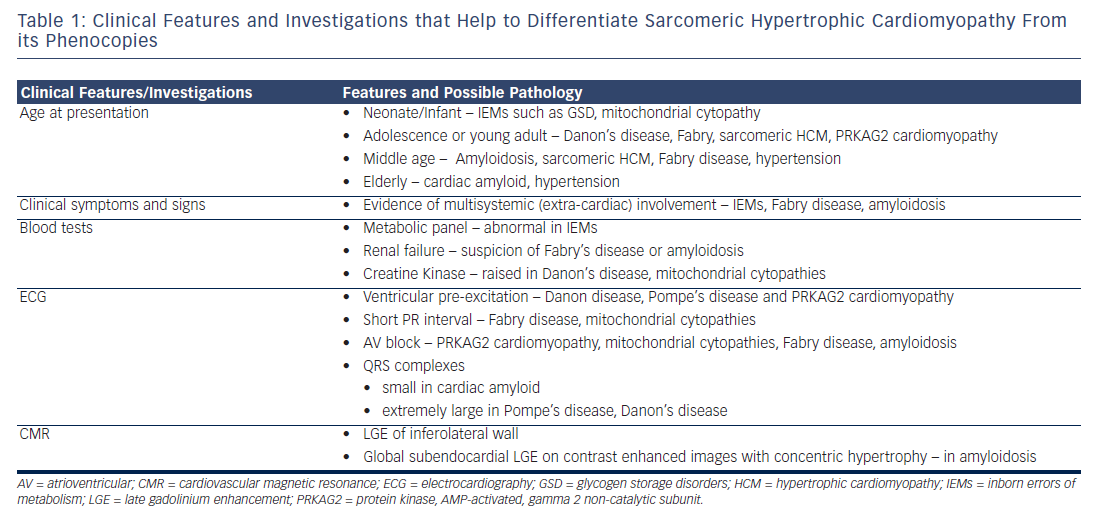
Despite the early onset of multisystemic manifestations of most HCM phenocopies, there is usually a significant delay in accurate diagnosis of these conditions and also a possibility that many of these are misdiagnosed as HCM due to sarcomeric mutations. As Table 1 illustrates, there are several ‘red flag features’ and presence of these or LVH in the absence of conventional diagnostic criteria for HCM, should alert the clinician to consider investigating for a HCM phenocopy. The natural history and management of these conditions differ from those of HCM due to sarcomeric mutations, thereby underlying the need for a high index of suspicion for these disorders, use of multimodal investigations and early referral to specialist cardiomyopathy clinics with access to detailed genetic testing, screening of family members and counselling in order to enable early diagnosis and appropriate management.








