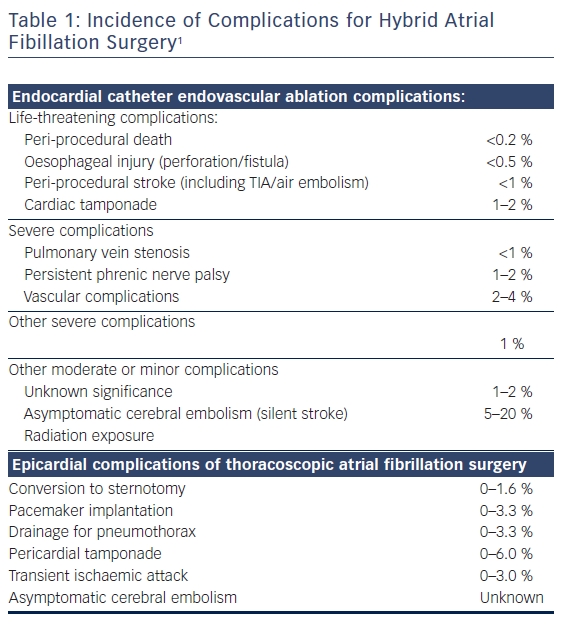
The current option for refractory treatment for atrial fibrillation (AF) includes hybrid AF-surgery.1–2 The hybrid approach was originally a combination of mini-invasive surgical epicardial evaluation and ablation, as well as endocardial electrophysiologist (EP) catheter ablation with the intention of creating a lesion set to cure AF.3 In the search for greater efficacy with less patient invalidation, different surgical and EP approaches have been developed. Variable timing between the epicardial approach and EP endocardial procedure has also emerged. Potential risks (Table 1) and claimed conversion rates to sinus rhythm after hybrid AF treatment vary from 27 to 94 %.4–13 The large variation in success rate is probably due to differences in patient selection between centres, staging and surgical technical approaches, lesion sets, periprocedural care and endpoints, energy sources, and choice of endpoints used after the procedure. Experience and technical improvements, as well as a search for greater value-based healthcare treatment of AF has led us to develop a one-stage hybrid method described in this manuscript.
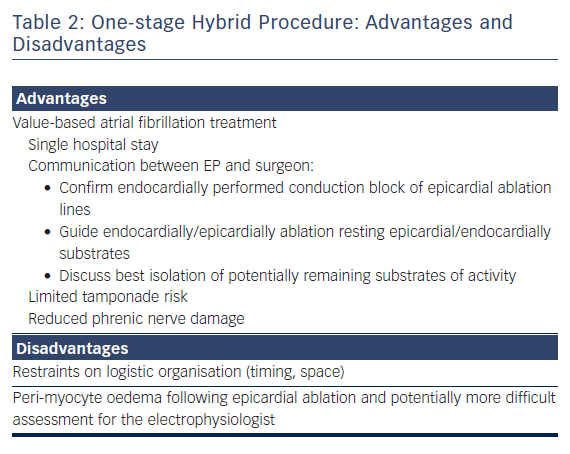
For refractory AF, our team recommends a combination of minimal invasive epicardial evaluation and ablation, together with EP mapping and ablation on the beating heart in a single procedure. The suggested epicardial technique involves mostly surgical unilateral left thoracoscopic evaluation and ablation approach, while the EP maps and ablates throughout the endocard after a percutaneous femoral approach during the procedure. Sometimes, depending on the patient’s antecedents, the epicard is approached with either a sequential, bilateral or unilateral right thoracoscopy.4 We believe that a one-stage approach provides unique and important advantages for patients compared to a two-stage procedure (Table 2).
The approach for hybrid AF surgery involves some particularities and challenging issues that require specific attention from the medical specialities involved. The purpose of this review is to describe our standard approach as follows: determine optimum timing of the last anticoagulant administration during the preoperative visit to minimise thrombus risk in the left atrial appendix and bleeding risk during surgery; expose respiratory management of prolonged sequential bilateral or unilateral thoracoscopy, hereby optimising oxygen delivery but reducing the risk of atelectasis; interpret haemodynamic control problems related to thoracoscopy, diastolic dysfunction with loss of atrial kick and decreased venous return by ablation on the pulmonary veins; show optimal positioning for surgery to facilitate access of the epicardial radio-ablation procedure; point out specific problems related to postoperative pain, its localization and treatment options; touch on the potential consequences of left atrial appendix clipping on fluid administration during and after surgery; and comment on early feeding problems after surgery.
Hybrid Method
Team
In practice, hybrid AF-surgery may be considered a joint venture of different medical specialities requiring close cooperation and immediate presence of a cardiologist, electrophysiologist, cardiac surgeon, anaesthesiologist, immediate care holders (nurses of the ward, operating room, high care or intensive care team, acute pain team), respiratory care specialists and pain doctors. The cardiopulmonary bypass department is also informed and on standby due to a <1.6 % risk of conversion to sternotomy with eventual repair under cardiopulmonary bypass.11
Patient Preoperative Visit
An in-depth preoperative visit focusing on the patient’s rhythm history and their cardiovascular risk factors often allows estimation of probability of conversion to sinus rhythm and duration of surgery. The factors affecting success rate are prior duration of AF, size of left atrial dilatation11 and prior treatment of comorbidity factors such as arterial hypertension, valvular heart disease, obesity, chronic obstructive pulmonary disease, chronic kidney disease and obesity before surgery.1
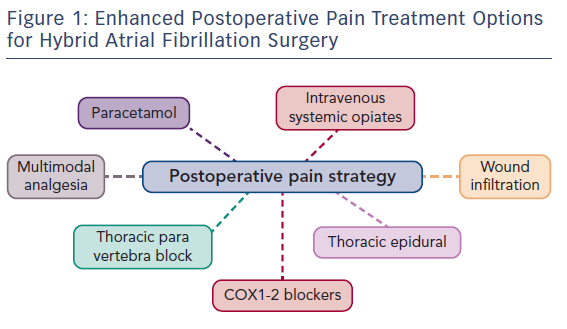
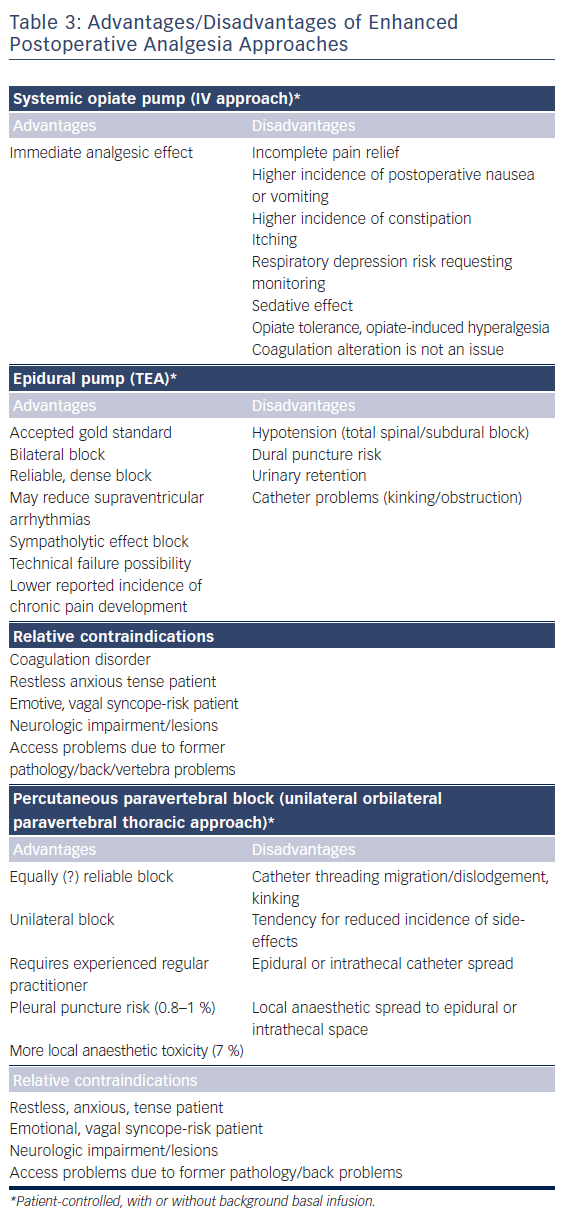
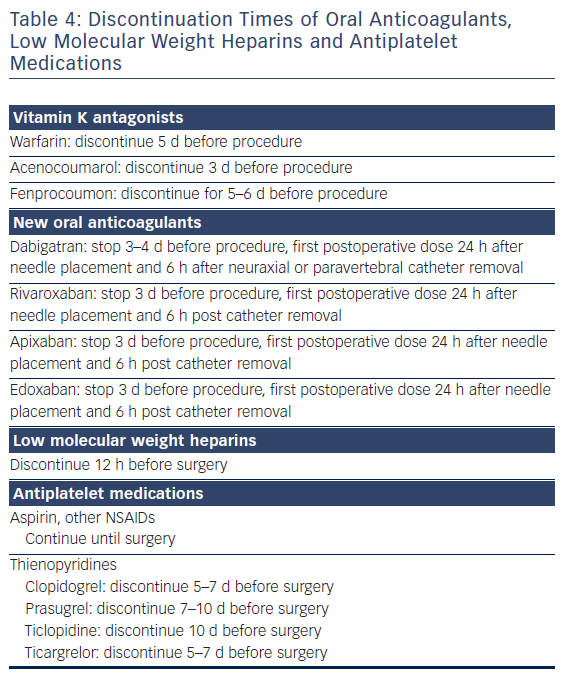
As surgery often requires sequential single-lung ventilation, anamnesis of former pulmonary afflictions is recorded. Postoperative choices of enhanced pain treatment after surgery, with consideration of multi-model therapeutic strategy14 (use of diverse analgesic compounds to reduce opiate consumption; see Figure 1) and particular choices should be discussed during the preoperative visit. The benefits and risks of treating postoperative pain using intravenous, epidural or paravertebral approaches are explained.15–20 Local anaesthetics with or without opioids are used for the epidural or paravertebral approach. A morphine or piritramide opiate pump is used for the intravenous route. A choice is made in light of the patient’s history and preferences, and experience of the anaesthetist (Table 3). Of concern in this choice is coagulation status just before surgery. The exact timing of discontinuation of anticoagulants before surgery is discussed in Table 4. Briefly, the aim is to find a window of opportunity between risk of bleeding with existing coagulation guidelines during surgery,21–25 and increased risk of thrombi generation in the left atrial appendix of patients with AF and an enlarged left atrium with decreased blood flow velocities. This is not always an easy task. Most guidelines for stopping anticoagulants apply to general surgery and not specifically to AF patients scheduled for hybrid AF surgery, where the left atrial appendix will be mobilised and ultimately stapled. If there is doubt of coagulation status, an assessment of the patient’s coagulation status just before incision with TEG/ROTEM monitoring, when available, may reduce the risk of epidural or paravertebral bleeding with subsequent medullar compression.26–28 However, the exact value of using this method of monitoring in this context is insufficiently studied and needs to be further investigated.29
A detailed, yet gentle explanation to patients about the goal and surgical technique is often helpful in reducing preoperative anxiety and sympathetic activity. In most cases, the patient’s preference is a patient-controlled intravenous or thoracic epidural technique. A paravertebral block is eventually used as a postoperative rescue issue for patients with refractory pain after patient-controlled intravenous pain when administration of non-steroidal anti-inflammatory agents is not indicated. Both epidural and paravertebral block with catheter insertion in AF patients under anticoagulant treatment present a threat challenge to the anaesthesiologist as inserting the needle or catheter may lacerate a blood vessel and subsequently create a risk of medullar compression.29 In normal circumstances the risk for neuraxial haematoma is estimated to be between 1 in 220 000 and 1 in 320 000. Short new oral anticoagulant (NOAC) discontinuation times may increase this risk. Multiple neuraxial puncture attempts should, therefore, be avoided in patients with spinal abnormalities or with other underlying hereditary or acquired coagulation disorders.
Patients are informed of the need for radial artery catheter for beat-to-beat blood pressure monitoring during surgery and insertion of a jugular venous catheter for eventual continuous administration of inotropic support during or immediately after surgery.
Anaesthetic Approach Before Surgery
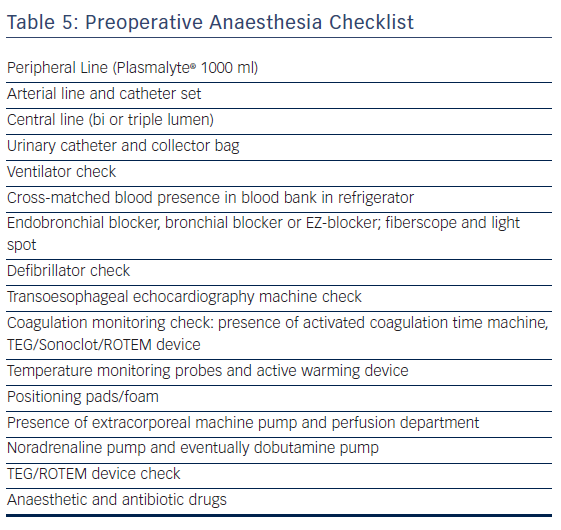
Preparation of anaesthesia before surgery (see the preoperative checklist in Table 5) includes radial artery monitoring set, eventual epidural catheter set, patient-controlled intravenous or epidural pump device, intubation equipment for left and/or right-sided thoracoscopy through selective left endobronchial intubation by endotracheal intubation with a bronchial blocker or EZ-blocker under fibrescopic control, central venous catheter set, norepinephrine, and possibly dobutamine pump. All patients receive a bladder catheter. Temperature recording with active patient warming (Bair Hugger or analogue) to maintain core temperature (bladder temperature) above 35.5°C is favoured. External and internal defibrillator pads and a same-day checked defibrillator are needed. The immediate vicinity of a primed cardiopulmonary bypass (CPB) machine is checked for possible repair of pulmonary vein or left atrium laceration under cardiopulmonary bypass.
Surgery
Anaesthetic drugs are determined by the patient’s antecedents and anaesthetist’s habits or preferences and include opioids, propofol/etomidate, non-depolarizing muscle relaxants (rocuronium, cisatracurium) and inhalational anaesthetics (isoflurane, sevoflurane). All patients receive antibiotic prophylaxis before incision. A repeat dose is given for surgeries lasting for more than 4 h.
Anaesthetic specificities for hybrid surgery involve control of perioperative haemodynamic and ventilatory stability and postoperative pain control.
The ventilation settings are adapted to single-lung ventilation request during surgery. For single-lung ventilation tidal volumes are decreased (from 7–8 ml/kg to 5–6 ml/kg ideal bodyweight), respiratory frequency increased (from 14 to 20/min or more depending on initial basal respiratory rate). High versus low positive end-expiratory pressure (PEEP)-levels, when needed, are prudently increased depending on their impact on blood pressure and pulse oxygen saturation. Often, a permissive attitude to hypercapnia with arterial carbon dioxide levels of 50 mmHg is accepted. Alveolar recruitment is performed depending on patient blood pressure, pulse saturation and surgical consent. Particular attention should be given to alveolar recruitment when changing sides if a two-stage sequential thoracoscopy is performed.
An enhanced incidence of hypotension due to diastolic dysfunction, loss of atrial kick, carbon dioxide insufflation in the thoracic, mediastinal and pericardial cavities, and effect of surgery during ablation on pulmonary venous return is frequently observed after onset of single-lung ventilation. Carbon dioxide insufflation pressure during thoracoscopy should be adapted to blood pressure and limited to 8 mmHg or less to avoid a tamponade effect. Judicious titration of phenylephrine, noradrenaline or dobutamine is often requested. Impaired or restrictive diastolic function, sequential one-lung ventilation with clipping of the left atrial appendage and consequent altered postoperative atrial natriuretic hormone levels justifies reasoned and restricted fluid administration.
Fluid administration is adapted to current guidelines30–32 and eventual surgical blood loss. As in most cases, blood loss is minimal and blood products can be avoided. Fluid administration starts with a balanced crystalloid solution set at 2 ml/kg per h and is further adapted in function of transoesophageal echocardiography imaging. However, a tendency to restrain fluids is observed in later stapling of the left atrial appendage. In general, total fluid administration given during surgery ranges from 500 to 1000 ml.
Patient Positioning
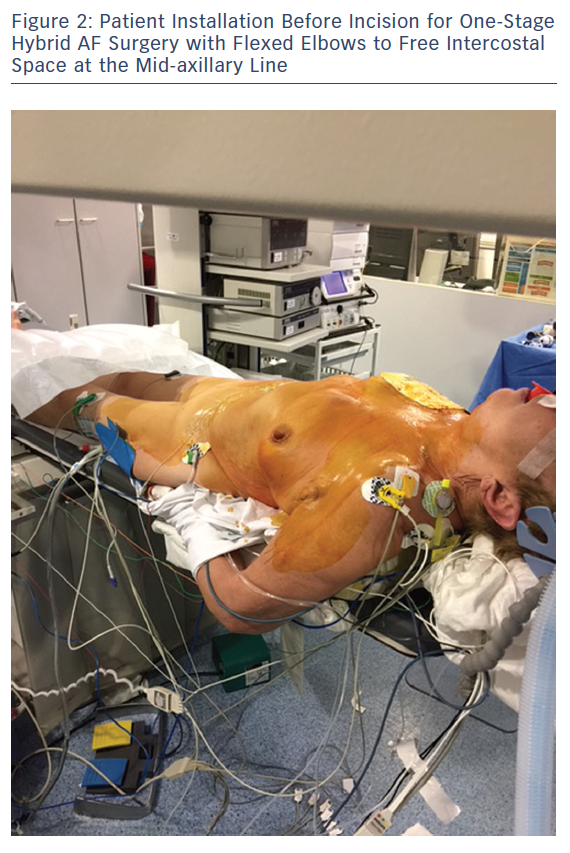
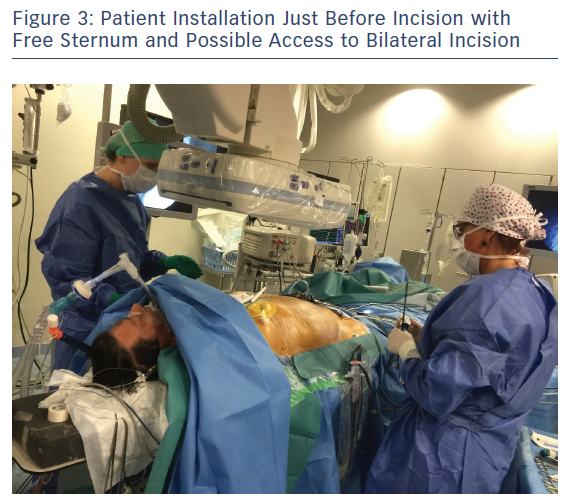
Patient positioning for surgery involves opening the space on the front axillary line to provide optimal access to the fourth and fifth intercostal space with an inflatable balloon placed below the patient’s back. The balloon is inflated for improved access and widening of the intercostal opening with consideration to the pressure exerted on the cervical lordosis and the possible hyperextension in the lumbar area. Both arms are flexed and placed sideways to the operation table to widen the operative area. A radial artery catheter is preferred as a brachial artery catheter often leads to greater kinking or obstruction in patients positioned with flexed elbows (Figure 2). The area surrounding the sternum is kept electrode-free for a worst-case scenario with unforeseen bleeding leading to sternotomy (Figure 3).
Epicardial Approach
Hybrid AF-surgery consists of a combined thoracoscopic epicardial and endovascular endocardial approach. The epicardial approach consists of left and possibly right thoracoscopy. Briefly, a 6 mm camera port is placed in the fifth intercostal space at the mid-axillary line and in the sixth or seventh intercostal space anterior axillary line. A 5 mm working port is placed in the third intercostal space anterior axillary line. The pericardium is opened anterior to the phrenic nerve and transverse and oblique sinuses, and is bluntly dissected. A bipolar RF-clamp is used for antral isolation of both pairs of pulmonary veins with four to six applications. Afterwards, a bipolar RF pen or linear pen device is used to perform a roof line and inferior line connecting, respectively, on both superior and inferior pulmonary veins to create the box lesion for posterior left atrial wall isolation. Box lesion ablation is completed when the right atrium is dilated with two additional ablation lines: one line with the use of the clamp encircling the vena cava superior and a bicaval line using the pen and connecting both caval veins. In most patients, the left atrial appendix appendage is also clipped. Communication between the surgeon and anaesthetist is important as pulmonary venous return is sometimes hindered during ablation. Subsequent hypotension and lowered oxygen saturation levels are treated with norepinephrine titration, higher inspired oxygen levels as indicated, and surgical repositioning.
Electrophysiology
Percutaneous femoral electrophysiological examination and ablation includes the insertion of a decapolar coronary sinus catheter under fluoroscopy and a trans-septal puncture for placing a long 8-Fr sheath under combined guidance of TEE and fluoroscopy. The patient is heparinised after the trans-septal puncture with a heparin dose of 1,000 IU/10 kg bodyweight to obtain a target activated clotting time >300s. Eventual heparin increments are given to maintain ACT values. A circular mapping catheter and a radiofrequency (RF) ablation catheter are used with an open, irrigated 3.5 mm tip to create a detailed electro-anatomic map of the left atrium. The ‘epicardial box lesion’ ablation block is controlled endocardially by checking the entry and exit block of the pulmonary veins alongside the posterior wall of the left atrium. The circular mapping catheter is used to evaluate the entry block of the pulmonary veins and the posterior left atrial box, and is defined as the absence of atrial bipolar signs. The exit block checks the pulmonary veins or the posterior wall during pacing with an output of 10 mA and a pulse width of 2 ms without conduction to the left atrium. If either block is not present, additional endocardial ablation is performed to close the conduction gaps. A radiofrequency power-controlled mode with a power limit of 35 W with maximal temperature of 48°C is used until block achievement.
A cavo-tricuspid isthmus endocardial or a mitral line ablation is added in the presence of a flutter or a mitral isthmus dependent flutter, respectively, during the procedure. If despite these epicardial and endocardial ablations AF persists, left and right continuous fractionated atrial electrogram mapping/ablation is performed. Target sites are defined as the fastest local repetitive electrical activity, multicomponent fragmented signals or as activation delay between the distal and proximal bipolar electrodes covering most the cycle length. The endpoint of ablation coincides with a regular or disappeared local signal, conversion to sinus rhythm or the presence of stable atrial flutter. Patients who do not convert to sinus rhythm during hybrid AF ablation are cardioverted. In sinus rhythm, all ablation lines are then revisited to confirm bidirectional conduction using the standard criteria. The pericardium is approximated with a stitch, and a chest tube is placed in one or both pleural cavities depending on the approach.
Perioperative Management
Pain Triggers
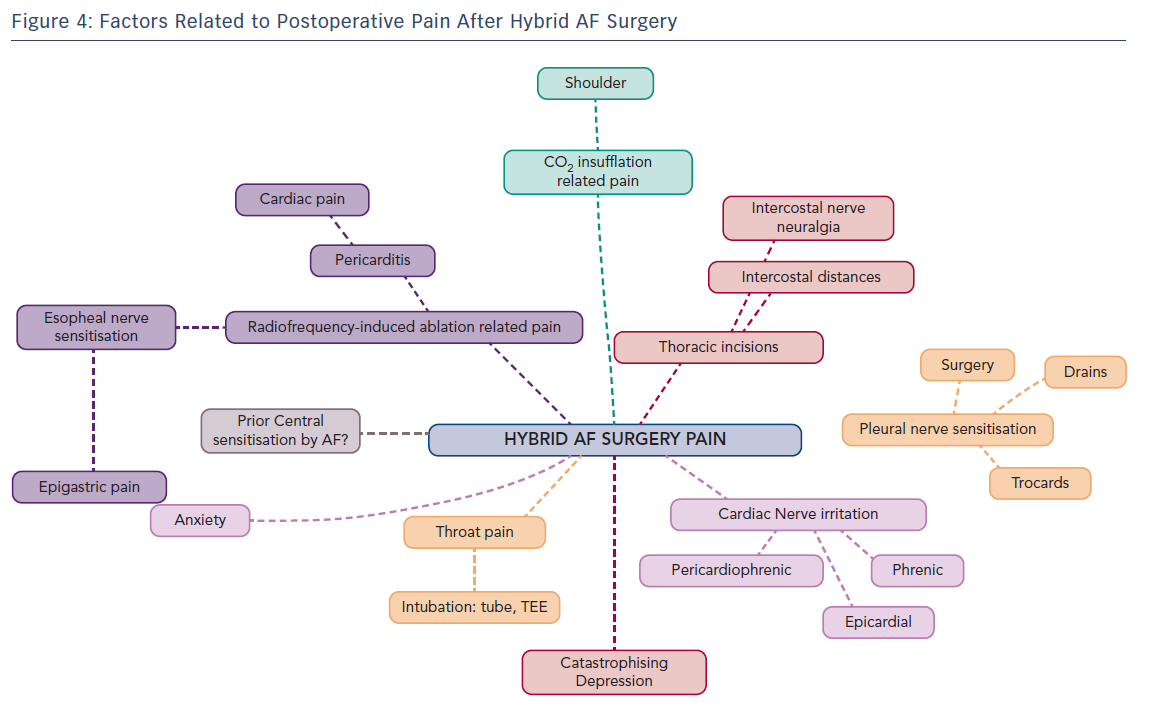
Postoperative pain treatment after hybrid surgery for AF presents interesting challenges with a fluctuating variability of localisation and intensity. This is explained by nerve sensitisation induced at several places by the surgical approach combined with the endocardial and pericardial radiofrequency-ablation procedure (Figure 1).
The first potential cause of postoperative pain, and often the first reported, is related to neural activation in the pleural cavities. This is caused by the insertion of intercostal ports, insufflation of carbon dioxide (which cannot always be totally removed at the end of surgery) or the presence of thoracic drains in one or both pleural cavities. Pain symptoms are specifically respiratory (inspiratory) and drain-related. In case of shoulder pain, inadequate CO2 removal with secondary phrenic nerve irritation should be considered. Of importance for the long term, the intercostal nerves may be pressurised by the 5-mm ports in patients with narrow intercostal distances and rarely progress to intercostal nerve neuropathic pain.
The second possible pain course is by neural activation and sensitisation of several (epicardial) heart nerve endings during the radiofrequency ablation approach. Possibly implicated and sensitised nerves are the phrenic nerve(s), pericardiophrenic nerve, epicardial nerve and intrinsic cardiac nerves, which regroup in a ganglionated plexus located in fat pads. At the atrial level three plexi are described: a superior left atrial plexus disposed over the superior veno-atrial junction, a posterolateral plexus laying over the lateral atrial wall and the left inferior veno-atrial junction and a posteromedial plexus overlying the right veno-atrial junctions. Signals from the heart are transmitted by primary sensory neurons. The afferent fibres originating from the heart travel as sympathetic and parasympathetic nerves and transmit information to the central nervous system about the activities of the heart or the occurrence of tissue injury, the latter through nociceptive fibres. These signals also generate autonomic reflexes, which allow regulation of organ function. Some of the signals, for instance pain signals, can be transmitted to cortical centres and become conscious. Nociceptive fibres are mostly associated with sympathetic fibres. Their cell bodies are found in spinal ganglia and likely end in the posterior roots of the spinal cord, where they synapse in second-order nociceptive neurons. Nociceptive visceral fibres are much less numerous than the nociceptive somatic fibres and usually end at more levels in the spinal cord, thus generating less specific and localised pain sensation. Afferent primary sensory fibres associated with parasympathetic nerve fibres are more involved in regulatory reflexes of system/organ activities. These nerve fibres are mainly associated with the vagal nerve, but several visceral cranial afferent fibres travel with the glossopharyngeal nerve or facial nerve and fibres of the pelvic nerves. The cell bodies of these afferent fibres are found in ganglia associated with these parasympathetic nerves. Primary sensory neurons then project onto second-order visceral sensory neurons located in the medulla oblongata, the nucleus of the solitary tract and other nuclei.33–34
Reported pain sensation after radiofrequency ablation can vary. Most often, a persistent dull type of cardiac pain is reported, but severe, sharp, diffuse heart pain is also possible. This type of pain may be associated with altered ST-segment elevations on ECG-recordings and troponin elevations, and is induced by regional pericarditis radiofrequency. This is to be differentiated from coronary embolisation of left atrial thrombus, ablation damage to the left circumflex artery and pericardial blood effusion. Pericarditis pain is treated with aspirin, NSAIDs, or colchicine when appropriate, often in combination with intravenous opioid administration.35 Whether preoperative central sensitisation induced by the presence of a long-standing AF, young age, extent of radiofrequency ablation or ongoing long-lasting anxiety potentiate this postoperative pericarditis pain is still unknown.
A third possible source of pain is of epigastric or oesophageal origin. Its causes are dual. The reported incidence of inflammation of the anterior oesophageal wall secondary to the RF-ablation on the antrum of the pulmonary veins is 47 %.36 This oesophageal inflammation may also be related, albeit to a lower extent, to TEE insertion and manipulation for exclusion of the presence of thrombi in the left atrial appendix just before surgery.37
Whether preoperative central sensitisation induced by the presence of a long-standing AF, young age, extent of radiofrequency ablation or ongoing and long-lasting anxiety, depression or catastrophising behaviour potentiate this severe postoperative pericarditis pain is unknown.
Establishing the difference between cardiac pain and stomach/oesophageal pain is sometimes difficult as both the posterior wall of the left atrium and the pulmonary veins share some common efferent nociceptive nerves. In our experience, its onset is later than pain reported that is related to pleural or pericardial neural sensitisation. Discrete oesophageal dysphagia or slow oesophageal food descent often mentioned with resumption of solid fluid intake may be part of this nervous sensitisation. A gastroscopy performed after surgery may help exclude pain of oesophageal or gastric origin.
Pain Control
Pain treatment in our institution starts with paracetamol given before incision and continued for 24–48 h. A surgical infiltration of the wound and the intercostal nerves with ropivacaine is accomplished before closure of the laparoscopic incision sites. Pain control is further enhanced with either an intravenous opioid or an epidural pump with local anaesthetics started 30 min before skin closure. The patient-controlled IV pump consists of either a morphine or piritramide pump. Depending on the VAS scales and patient’s complaints, a background continuous infusion is eventually added. Most pain complaints after surgery decrease drastically after thoracic drain removal. In some patients, however, pain persists for another week. Further pain relief is provided when possible or indicated with intravenous NSAIDs, selective COX-2 inhibitors, tramadol, or morphine/oxycodone. When pain treatment is refractory a paravertebral block is eventually performed.
Postoperative Management
Cardiac Management
Patients are monitored for eventual arrhythmia relapses, coronary vasospasm, circumflex coronary artery damage by radiofrequency and coronary thrombus embolisation for 24 h in intensive care.
For patients with suspected RF-induced pericarditis, regular ECGs and troponin measurements are performed. After ICU discharge some patients are placed under continuous telemetric monitoring. Transthoracic echocardiography is requested and performed to exclude post-procedure pericardial effusion. Low molecular weight heparins are started the evening after surgery. NOACs and oral anticoagulants are re-initiated on the third postoperative day and continued for 3 months. The left atrial appendix is stapled to reduce the stroke incidence after hybrid AF surgery.38–39 Closure of the left atrial appendix leads to lower atrial natriuretic peptide levels and consequently higher risk of fluid retention in the immediate postoperative days. Careful postoperative fluid administration with regular patient weighing is therefore indicated in the first weeks after surgery. A one-month diuretic treatment is usually used to bridge the period before the right atrial appendix or the brain compensates with the patient’s personal natriuretic peptide production at our hospital.40
Follow-up
Clinical follow-up by the cardiologist and/or surgeon with a physical examination, electrocardiogram and 24-h Holter monitoring is implemented at 3, 6 and 12 months following hybrid surgery. A gentle cardiac rehabilitation program following the procedure is encouraged.
Conclusion
Hybrid AF-surgery is a promising therapy for patients refractory to AF-treatment. An integrated approach involving different teams may lead to improved success rate and increased patient satisfaction. Differentiation of the several possible pain triggers, whether thoracic, cardiac or gastro-esophageal in origin, can help resolve observed versatile postoperative pain.