Radiofrequency (RF) catheter ablation is currently the treatment of choice in patients with accessory pathways (APs) and Wolff– Parkinson–White syndrome, and is shown to have a success rate >95 %.1 APs usually have endocardial ventricular and atrial insertions, located close to the atrioventricular valve rings, making most endocardial catheter ablation procedures relatively straightforward and yielding a high success rate. However, this is not the case when the AP distal end is closer to the epicardial surface or its atrial or ventricular insertion is located far from the atrioventricular groove, and a small subset of patients will fail ablation procedures using a conventional endocardial approach.2,3
Endocardial Ablation
Endocardial catheter ablation has limitations, including the inability to access intramural or epicardial portions of arrhythmia circuits. Epicardial AP location was pointed to as the cause of 8 % of prolonged and failed AP ablation attempts.4 Technological improvements, such as cooled-tip, larger-tip ablation catheters, contact-force technology and different energy sources for tissue ablation have not completely solved the problem, and some arrhythmia substrates might not be accessible from the endocardium.5
Several other factors may contribute to RF ablation failure: difficulties with catheter manipulation, including an inability to reach the appropriate AP site, catheter instability (particularly in right-sided AP) or inadequate tissue contact; inaccurate mapping related to AP slanting and AP localisation away from an endocardial-positioned catheter or in the setting of Ebstein’s anomaly; proximity of the AP to vital structures, such as a coronary artery or the atrioventricular node; associated structural abnormalities, such as congenital venous system anomalies or acquired coronary system stenosis that has developed as a consequence of previous unsuccessful ablation attempts.4
Some of these difficulties can be overcome during cardiac surgery (open-chest surgery or thoracoscopy), an epicardial approach performed through epicardial vessels of the coronary sinus (CS) system or through percutaneous catheterisation of the pericardial space, as described by Sosa et al.5,6
Intravenous Mapping and Ablation
AP located in the posteroseptal and left posterior areas may be difficult to ablate due to relative epicardial localisation, thickness of the myocardium, anatomic complexity of this area and coexistence of a CS diverticulum, containing a pouch and neck.7,8 CS anatomy should be carefully assessed, either by venography or CT, to rule out diverticulum, which may be present in 15–20 % of refractory posteroseptal APs. Cooled-tip catheter ablation inside the CS venous system and middle cardiac vein is effective in most epicardial posteroseptal APs. However, one has to be aware that a fast conducting AP may become a decremental AP after an ablation attempt. In this instance, the ECG may change, lacking overt preexcitation during sinus rhythm. Its correct identification is possible if a thorough programmed electrical stimulation is carried out after the ablation attempt.3,4,8,9
In 1992 Haïssaguerre et al. reported the effectiveness and safety of radiofrequency catheter ablation of left lateral APs via the mid or distal CS when endocardial approaches are unsuccessful.10 They had no significant complications, except a marked nonspecific pain during RF energy application.10 In 1993 Langberg et al. evaluated a group of patients with left-sided APs that were difficult to ablate from the endocardial surface. It was found that the absence of an AP potential during endocardial mapping in combination with a relatively large AP potential within the CS may be a useful marker of a subepicardial pathway localised in the atrioventricular groove. In this select group of patients, radiofrequency application from within the CS appears to enhance ablation efficacy.11
Morady et al. reported on a series of difficult catheter ablation cases: in three patients who were initially thought to have a right or left posteroseptal AP, the effective target site was 2–3 cm within the CS or within a posterior interventricular branch of the CS. In two patients thought to have a left lateral AP, the AP site was mapped within the CS in the region of the lateral mitral annulus. In each of these patients, AP potentials were absent or small in amplitude from the endocardium, but a relatively large potential was recorded within the CS.4
The CS has a myocardial coat with extensive connections to the left and right atria. An extension of this coat through the posterior coronary vein, the middle cardiac vein or a diverticulum neck can connect to the left ventricular epicardium and form epicardial posteroseptal and left posterior AP.1,12 CS APs (defined by earliest activation within the venous system) were identified in 36 % of patients with posteroseptal or left posterior AP in a study of a select group of patients where most had failed previous attempts at ablation; the actual incidence of such pathways should be much smaller.12 Usually CS APs have an oblique course because of the oblique orientation of the fibres connecting the CS myocardial coat with the left atrium.13 CS angiography revealed a CS diverticulum in 21 % of patients and fusiform or bulbous enlargement of the small cardiac vein, middle cardiac vein or CS in 9 % of patients.12 These venous anomalies mostly arise 1.5 cm away from the CS and before the middle cardiac vein, but they can originate from the middle or posterior cardiac veins as well.1 Successful ablation of these pathways may be achieved while ablating in the diverticulum neck.1,14
A precise knowledge of the CS anatomy and its potential abnormalities, such as the presence of diverticulum or persistent left superior vena cava, as well as CS electrogram recordings, are essential for successful RF catheter ablation in patients with a prior history of multiple ablation failures or in whom successful ablation cannot be achieved.1 The presence of a negative delta wave in lead II is suggestive of an epicardial localisation of the AP (identifying a CS AP), with a sensitivity of 70 %.1 Takahashi et al.15 reported that the combination of a steep positive delta wave in lead aVR and a deep S wave in lead V6 (R wave ≤ S wave) during maximal pre-excitation had the highest specificity for identifying epicardial coronary vein posteroseptal APs, while the highest sensitivity is provided by a negative delta wave in lead II.
Ablation in posteroseptal diverticula has lower success rates and is correlated with more procedural complications due to the close proximity of the epicardial coronary arteries, risk of venous perforation, tamponade, venous occlusion or heart block.1 Success can usually be improved by targeting the neck of the diverticulum, applying irrigated-tip catheters, using cryoablation or performing the subxiphoid epicardial approach.16 Although RF ablation can be done safely inside the CS, cryoablation could be a safer alternative, especially if the best ablation location is in close proximity to a coronary artery, although a higher rate of recurrences have been reported.9,17
An alternate method for mapping right-sided APs that did not stand the test of time involved the placement of a multipolar mapping catheter within the right coronary artery. In many instances, the right coronary artery is located away from the annulus and therefore provides only a limited anatomic area for mapping compared with percutaneous epicardial mapping. These multipolar 2-F or 3-F mapping catheters are no longer available. The rationale for this approach was analogous to the placement of a multipolar catheter within the CS for mapping left free-wall pathways.6
Percutaneous Epicardial Mapping and Ablation
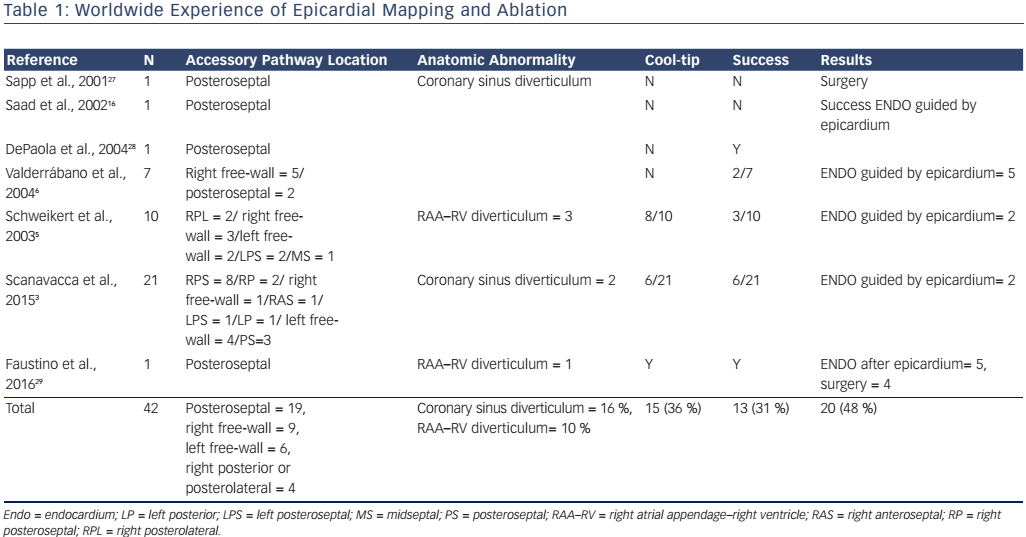
There are some case reports and a few series of cases about using the epicardial percutaneous subxiphoid approach to map and ablate APs (see Table 1).3
Pericardial Access
Access to the epicardial space was achieved as previously described by Sosa et al.18 A subxiphoid transthoracic epicardial puncture was performed using an epidural needle. As the needle was advanced, radiographic contrast was injected to confirm entry in the epicardial space, allowing the introduction of a J-tipped wire. Absence of needle entry in the right ventricle was demonstrated by advancing a guidewire along the left heart border in the left anterior oblique view and by aspirating amber pericardial fluid. A standard sheath then was advanced over the wire. A long and deflectable sheath was substituted and advanced into the transverse sinus of the pericardium as needed to improve catheter stability.6
Clinical Experience
In 2003 Schweikert et al. reported a series of previously failed catheter ablations in 48 patients who were subjected to combined epicardial–endocardial mapping. This series included 10 patients with AP-mediated tachycardia. In three of these cases, successful epicardial ablation of right atrial appendage–right ventricle epicardial APs was achieved. Of the other seven cases, epicardial mapping yielded earliest activation only in two cases, and ablation was ultimately successful from the endocardium but not from the epicardium.5
Cases of right atrial appendage–right ventricle AP have been described, although they represent rare situations (see Table 1).5,19–21 Misidentification of the AP location is not uncommon and successful ablation may have to be performed far from the annulus, at the atrial appendage insertion site, which is the site of the earliest ventricular activation.18 However, the atrial appendage is a difficult target for ablation, even using irrigated catheters, due to limited blood flow between the catheter and the trabeculated surface of the appendage.20 When endocardial ablation fails, a percutaneous epicardial approach has been demonstrated to be safe and effective in several case reports and can be considered an alternative to surgery.5
Left atrial appendage–left ventricle APs have recently been reported. Di Biase et al. described two adult patients with APs involving the left atrial appendage which were difficult to ablate with conventional catheter techniques.22 Mah et al. reported three paediatric patients in whom this AP was impossible to ablate percutaneously, ultimately requiring surgical intervention.23 Catheter ablation failure is likely due to the broad-based nature of the connection (requiring extensive surgical dissection) and the close proximity of the left atrial appendage to major coronary artery branches.23
In 2004, Valderábano et al. aimed to define the role of percutaneous epicardial mapping in six consecutive patients (with seven APs) referred for catheter ablation after previous attempts had failed. Endocardial and epicardial mapping were performed to identify optimal target sites for ablation. Whenever feasible, the endocardial catheter was positioned across from the epicardial catheter to compare electrograms. Epicardial RF delivery was performed only when electrograms showed that the APs were in the best epicardial sites and after endocardial RF delivery had failed. In this series, the most attractive target site for ablation was epicardial in three of the six patients, and an epicardial RF application was necessary for successful ablation in two of these patients.6
In 2015 we reviewed 21 patients referred for percutaneous epicardial AP ablation after a median of more than two previous procedures had failed.3 All patients underwent a simultaneous endocardial and epicardial approach. In six patients (28.5 %) epicardial activation was found earlier than endocardial activation and they underwent successful ablation from the epicardium. In three patients, simultaneous early activation at the epicardium and endocardium close to the mitral annulus was seen, and two of these patients were successfully ablated from the endocardium, guided by epicardial mapping. In nine patients endocardial activation was earlier than epicardial activation and in five of them subsequent endocardial or epicardial transvenous mapping and ablation resulted in AP elimination. Thus, subsequent endocardial or epicardial transvenous mapping and ablation resulted in AP elimination in seven patients (33 %). In three cases no early signals were found from endocardial or from epicardial activation.3
A percutaneous epicardial subxiphoid approach should be considered when endocardial (or transvenous) mapping fails to identify a suitable ablation target or if ablation from the best site is unsuccessful, as:
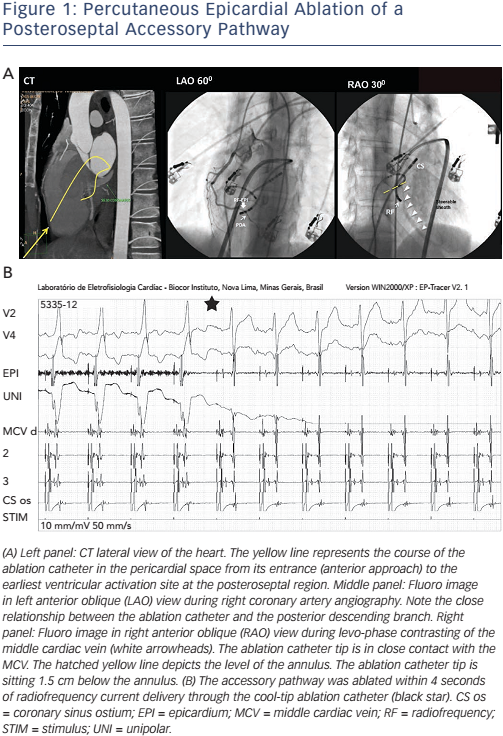
- Epicardial mapping can find a true epicardial AP, where ablation is successful (see Figure 1A and B). When the AP is sub-epicardial, as is the case of right atrial appendage–right ventricular diverticulum, an epicardial (percutaneous or surgical) approach may be the only possibility.3 The percutaneous epicardial ablation success rate is only between 28 and 33 %, however, according to different series.3,5,6 One reason is the epicardial fat – which is thicker in the vicinity of the atrioventricular annulus, covering the region where AP sits – that hampers AP ablation.5 Proximity to a major coronary artery may be another obstacle precluding epicardial AP ablation, due to safety issues. Myocardial tissue and APs that are located underneath large epicardial arteries frequently remain intact after ablation.24
- Epicardial mapping can guide and enhance the effectiveness of endocardial ablation. The identification of an early epicardial activation site works as a reference for successful endocardial ablation; patients with similar endocardium and epicardium activation times can successfully undergo endocardial ablation, according to data from the series previously presented.3 The epicardial approach allows easier and more complete mapping of the atrioventricular annulus without the anatomical restrictions of catheter manipulation from the endocardium. This approach also avoids distortion of epicardial electrograms from previous endocardial ablation attempts.6
- The finding of no early epicardial activation should lead to a more intensive and persistent endocardial attempt. In our series, five patients were successfully ablated after a further attempt at the endocardial approach (including the endocardium and coronary venous system) following epicardial mapping. We hypothesise that when pericardial mapping identifies no adequate target, the operator makes a greater effort as he or she realises that the endocardial approach is the only possibility of success.3
- When no epicardial or endocardial site with early activation is found to allow successful ablation, open-chest surgery is the only option to eliminate the AP, particularly for high-risk patients. It is important to be sure that no early activation is present during epicardial percutaneous mapping and to repeat endocardial mapping. An irrigated-tip ablation catheter should be used as it may improve results, especially in patients with coronary venous system-associated lesions. Congenital anatomical anomalies, such as CS diverticulum, venous stenosis and ostia atresia, are associated with a higher probability of requiring surgery.3
Complications and Limitations
Although generally a safe procedure, subxiphoid percutaneous epicardial ablation of APs, like epicardial ablation of other arrhythmia substrates, may result in complications. Coronary injury is a matter of special concern. The procedure has the potential to damage epicardial vessels. This may occur while gaining access with the epidural needle, may be caused by the tip of the sheath or may occur during the delivery of epicardial radiofrequency current. Coronary angiography is the gold standard method for assessing the distance from the ablation site to a major coronary artery.5
Stavrakis et al.25 assessed 240 patients with an epicardial posteroseptal AP who had undergone ablation within the coronary venous system. The risk of coronary artery injury with radiofrequency ablation was inversely correlated with the distance between the coronary artery and the ablation site. Injury was observed in 50 %, 7 % and 0 % of patients when RF was performed within 2 mm, 3–5 mm and >5 mm of the coronary arteries. Cryoablation was found to be safe. No coronary lesions were reported, even when cryoablation was applied within 5 mm of the coronary artery.
A potential advantage of the percutaneous epicardial approach is avoidance of the endovascular complications that might be encountered with conventional endocardial techniques, such as vascular injury, valve damage and embolism from coagulum or dislodged plaque during left-sided ablation procedures. Ventricular fibrillation was reported to have occurred after coronary vasospasm during catheter manipulation in one case and after severe pericardial bleeding caused by middle cardiac vein laceration in another patient.26 Another advantage is that the use of intravenous heparin, and its associated complications, could be avoided.5 A potential limitation of percutaneous pericardial instrumentation is that it should not be used in patients who have undergone prior cardiac surgery, as postoperative pericardial adhesions could limit access to the pericardial space.5
In our series,3 we did not have any major complications. In the two patients in whom the right ventricle was inadvertently punctured, pericardial haemorrhage was immediately recognised and drained, without any further complications. However, one must be aware that unusual complications may also occur, such as intra-abdominal bleeding due to puncture of the liver, hepatic haematoma, right ventricle–abdominal fistula, and right ventricular pseudoaneurysm.26
Clinical Perspective
- A significant number of failed ablations with standard endocardial ablation methods might represent an epicardial arrhythmia substrate.
- The epicardial substrate can be approached percutaneously through the cardiac venous system or through subxiphoid percutaneous epicardial access.
- Percutaneous mapping in the pericardial space facilitates a successful outcome by improving the accuracy of endocardial mapping and subsequent endocardial ablation; percutaneous epicardial ablation has been successful in a minority of patients in whom this approach has been attempted.
- Pericardial instrumentation is safe when performed by an experienced team.
- A subset of patients may require open-chest surgery.