Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice.1 It has been estimated that >3 million people in the US and >4.5 million in the EU have paroxysmal or persistent AF.2–4 AF is associated with an approximately fivefold increased risk of stroke,5 threefold risk of heart failure,6 diminished quality of life7 and increased healthcare costs.8,9 Ventricular rate control strategy has been a traditional front-line and well-tolerated therapeutic option for the management of patients with AF. Although seemingly preferable in terms of potential advantages related to improved cardiac function and avoidance of unfavourable atrial electrical and mechanical remodelling, the currently available rhythm control options have important limitations. Antiarrhythmic drugs are considered first-line agents for rhythm control and the currently available drugs have limited efficacy in terms of achievement and maintenance of sinus rhythm (SR). Even in a clinical trial with particularly favourable results associated with the use of antiarrhythmic drugs, AF recurred in about 35 % of the patients in the amiodarone arm; the rates of recurrence of AF were even higher with the use of sotalol and propafenaone (63 %).10 Recent data from the Permanent Atrial fibriLLAtion Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS) trial and other studies on the utility of dronedarone as a newer antiarrhythmic agent also suggest its limited efficacy and significant potential for adverse events.11–13
Catheter ablation, although generally superior to antiarrhythmic drug therapy in achieving SR, still remains limited by non-trivial recurrence rates for AF as well as the potential for procedural complications and the lack of currently available data on very long-term efficacy and mortality.14–18 In the meantime, it continues to be an area of intensive research in terms of its role as an evolving therapeutic option to achieve sinus rhythm.
In this review article we aim to discuss recommendations for ventricular rate control strategy in the management of patients with AF based on currently available data and the relevance of a rhythm control strategy with the advent of recent treatment advances in the field of antiarrhythmic drug therapy and catheter ablation.
Rate Control
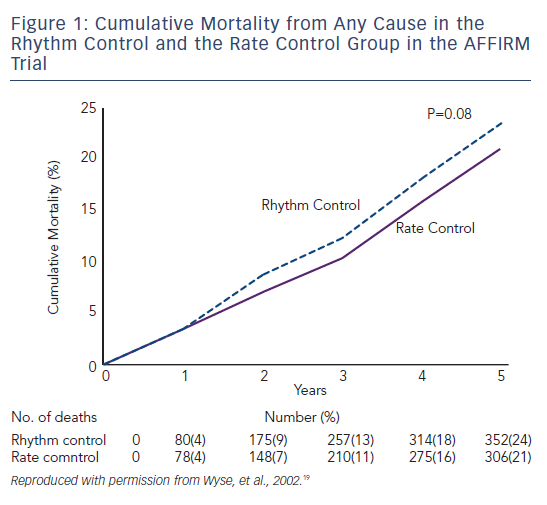
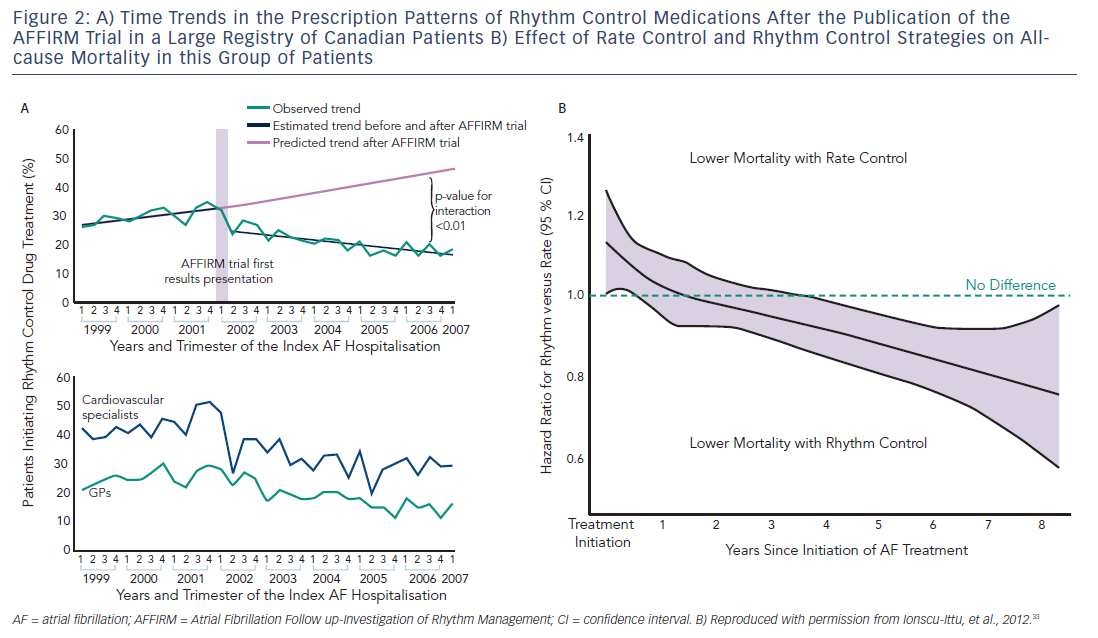
Ventricular rate control has been an area of continuous investigation over the years. Until the results of Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial were published, rhythm control was widely believed to be a superior therapy with the rationale of SR being associated with better exercise tolerance, superior symptom control and possible decrease in morbidity (see Figure 1).19 Despite these potential benefits of rhythm control, rate control was found to be non-inferior to rhythm control in the AFFIRM trial, with a trend toward reduced mortality with rate control. Results from the Rate Control Versus Electric Cardioversion for Persistent Atrial Fibrillation (RACE) study also confirmed that a rate control strategy is as effective as rhythm control in management of AF and offers potential advantages such as a lower risk of adverse drug effects, cost-effectiveness and decreased incidence of hospitalisations.20,21 Various meta-analyses have shown that a rate control strategy is at least as effective as rhythm control in patients with AF when comparing endpoints such as cardiovascular and all-cause mortality.22–25 It should be noted, however, that the majority of patients in these trials were elderly patients without highly symptomatic AF. A recently published meta-analysis of multiple randomised clinical trials comparing rate versus rhythm control strategies concluded that the incidence of stroke, systemic embolism, heart failure and myocardial infarction were similar between the two groups. The rhythm control strategy was found to be associated with a generally higher incidence of hospitalisations as compared with rate control. This could be potentially attributed to several factors such as necessity for achieving rhythm control by cardioversion in a monitored setting, adverse effects and arrhythmias secondary to the use of antiarrhythmic drugs.26 In a prospective cost-efficacy analysis on patients enrolled in the How to Treat Chronic Atrial Fibrillation (HOT CAFÉ) trial, a rhythm control strategy was found to be associated with increased overall healthcare costs and resource utilisation. This was attributed predominantly to the hospitalisations related to cardioversion, which generated not only direct procedure-related costs but also consultation costs.27,28 The trend of increased hospitalisations with a rhythm control strategy was also seen in other major trials (AFFIRM, RACE and Pharmacological Intervention in Atrial Fibrillation [PIAF]).19,20,29 Following the publication of these favourable results for rate control over rhythm control, there was a measurable increase in the prescription of rate control medications and decline in the use of rhythm control therapies for the management of AF (see Figure 2A). 29–33
Atrioventricular (AV) node ablation followed by permanent pacemaker implantation remains a viable treatment strategy for AF associated with difficult to control rapid ventricular rates. It was investigated by Brignole et al. in two randomised clinical studies involving patients with severely symptomatic paroxysmal AF, and patients with chronic AF and heart failure, respectively.34,35 This strategy principally relies on the fact that AV node ablation is very effective in controlling the ventricular response to AF, especially in patients who do not respond well to the pharmacological rate control strategy. AV node ablation has been demonstrated to be highly effective in controlling AF symptoms, improving quality of life and general wellbeing.36–39 The use of this strategy is further supported by its impact on decreasing healthcare costs, which are accounted for by a significant decrease in hospitalisations, outpatient visits and antiarrhythmic drug use.40
Despite these favourable results of AV nodal ablation and pacemaker implantation in patients with refractory AF, it is important to be cognisant of its limitations. In patients with paroxysmal AF who exhibit variable periods of SR, a single chamber (ventricular) permanent pacing system may lead to worsening of functional status due to the loss of AV synchrony and abnormal ventricular activation. In such patients who may have self-limiting episodes of AF, a dual-chamber, rate-modulated (DDDR) pacemaker with an autonomic mode switch capability would be more useful so that in the absence of AF, AV synchrony is maintained.41,42 The clinical studies by Brignole et al. also concluded that patients with chronic AF and co-existing heart failure might benefit from Ventricular Rate-modulated Pacing (VVIR) mode of pacing.34,35 Concerns raised by the results of the Dual chamber and VVI Implantable Defibrillator (DAVID) study regarding the role of right ventricular pacing as a causative factor in increasing the risk of heart failure admissions and the superior outcomes of biventricular pacing demonstrated by the left ventricular-based cardiac stimulation Post AV nodal ablation Evaluation (PAVE) study favours the use of biventricular pacemaker in patients with impaired left ventricular function, which is not attributed to tachycardia who undergo AV nodal ablation.43,44 Another limitation of this strategy is the lack of elimination of the thromboembolic risk because AF is still maintained in such patients. Finally, AV nodal ablation typically leaves patients pacemaker-dependent, and failure of the pacing system in these patients, although rare, can be potentially catastrophic.
Rhythm Control
Despite the therapeutic convenience and potentially lower adverse events associated with the rate control strategy, there are several arguments in favour of a rhythm control strategy. Although none of the major randomised controlled trials (RCTs) comparing rate control versus rhythm control demonstrated a net benefit of rhythm control in patients with AF, a recent, large population-based registry of 26,130 Canadian patients suggested superiority in overall mortality with the use of rhythm control strategy after several years of follow-up (see Figure 2B).33 In another recently published observational study, rhythm control was found to be associated with lower rates of stroke and transient ischaemic attack (TIA) in patients with AF who were at moderate and high-risk of stroke.45 Whether these registry studies have identified real and important advantages of medical rhythm control versus rate control, which were not found in the RCTs, or whether the registries were confounded by the inherent limitations of non-randomised study design, remains unknown. A meta-analysis of various RCTs comparing rate control with rhythm control demonstrated that rhythm control was superior to rate control for prevention of all-cause mortality in patients younger than 65 years.26 This difference in the results of RCTs and observational studies could be potentially explained by multiple factors, including underrepresentation of younger patients in the RCTs, and unidentified differences in co-morbidities in patients in the population-based observational studies. The follow-up period of patients in the observational studies was comparatively longer than the RCTs, which could be yet another contributing factor to the discrepancies in these results.
In the Atrial Fibrillation and Congestive Heart Failure (AF-CHF) trial, which was designed to investigate the effect of underlying rhythm on the quality of life and functional capacity in patients with AF and congestive heart failure, a higher proportion of time spent in SR was associated with gains in functional status and improved quality of life, although the rhythm control strategy was not found to be superior to rate control in this study.46 To further support this concept, a post hoc analysis of the AFFIRM trial demonstrated that the presence of SR (as opposed to a rhythm control strategy) was associated with a lower risk of death, while the use of rhythm control drugs was associated with worsened mortality, and these two effects appeared to counterbalance, resulting in roughly similar mortality for rate versus rhythm control.47 Similar results were also observed in the Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) trial, which showed that patients who had SR either with or without antiarrhythmic drug therapy had a better prognosis compared with patients who continued to have AF.48
The results of RCTs comparing rate control with rhythm control should therefore not be interpreted as demonstrating a lack of benefit of sinus rhythm, but rather that the currently available antiarrhythmic medications are limited in their efficacy and have adverse effects, which make their routine use as first-line therapy a less favourable therapeutic option for many AF patients, particularly those with minimal symptoms when treated with adequate rate control. These data also suggest that younger patients who were not well-represented in the RCTs may derive greater benefit from a rhythm control strategy, likely by prevention of long-term complications of AF. The concept of ‘AF begets AF’ can be kept in mind while making decisions for such patients, and the early period of paroxysmal AF may represent a window of opportunity to establish SR in these patients.49–51 The presence of AF not only predisposes to an increased risk of systemic thromboembolism but also has been demonstrated to be an important risk factor for endothelial dysfunction and inflammation, which can be potentially reversed by restoration of SR.52–55 Although a rate control strategy might be simpler to achieve in comparison to rhythm control, it generally will not prevent the progression of paroxysmal to persistent AF.56,57 This could be attributed to a potentially beneficial role of rhythm control to reverse the electrical and structural remodelling responsible for initiation and perpetuation of AF.58–60
A risk stratification scheme HATCH score (Hypertension: one point, Age >75 years: one point, TIA or Stroke: two points, Chronic obstructive pulmonary disease: one point, Heart failure: two points) has been developed to predict progression of paroxysmal to persistent AF. This score may be used for an early selection of patients for a rhythm control strategy in an effort to prevent disease progression. If a high score is found, then clinicians should recognise that long-term sinus rhythm maintenance will be more difficult to achieve, and rate control may be more appropriate.61 Development of safer antiarrhythmic drugs with improved efficacy remains an elusive target for the pharmaceutical industry, but would greatly aid in expansion of rhythm control strategy to a diverse patient population. Catheter ablation remains an alternative rhythm control strategy, which substitutes the upfront procedural risks for the long-term risk of antiarrhythmic drugs.
In the recently published ThermoCool-AF trial involving patients with paroxysmal AF, catheter ablation was demonstrated to be clearly superior to antiarrhythmic drugs for the endpoints of overall rhythm control, freedom from symptoms and overall quality of life.62 In regard to hard endpoints such as mortality and stroke, the ongoing multicentre Catheter Ablation versus Antiarrhythmic drug therapy for Atrial fibrillation (CABANA) trial will provide crucial data on the efficacy and safety of catheter ablation for achieving rhythm control and further aid in the treatment decision of rate versus rhythm control.63,64
Targets for Rate Control
Ventricular rate control in patients with AF has dual advantages – reduction in symptoms and improvement in cardiac efficiency. Uncontrolled ventricular rates have multiple adverse effects such as worsening of symptoms, decrease in stroke volume and development of tachycardia-induced cardiomyopathy and congestive heart failure.65,66 In the AFFIRM trial, adequate rate control was defined as an average heart rate ≤80 beats per minute (bpm) at rest and a maximum heart rate ≤110 bpm during either a six-minute walk or moderate exercise,19 whereas the standard for rate control in the RACE trial was more lenient, requiring only a resting heart rate <100 bpm. Analyses of the RACE and the AFFIRM trials suggested that the subgroup of patients with resting heart rates above 100 bpm had worse outcomes compared with patients with slower rates, but it was not clear whether this was due to the impact of better rate control, or reflected co-morbidities resulting in both higher heart rates and worse outcomes. In the subsequent randomised Rate control Efficacy in Permanent Atrial Fibrillation (RACE II) trial; a lenient rate control strategy (resting heart rate <110 bpm) was demonstrated to be as effective as strict rate control (resting heart rate <80 bpm and heart rate during moderate exercise <110 bpm) for the composite outcomes of cardiovascular mortality, hospitalisations for heart failure, stroke, systemic embolism, bleeding and life-threatening arrhythmia.67 In a retrospective analysis of four studies involving AF and heart failure patients with cardiac resynchronisation therapy defibrillator devices, a poorly controlled heart rate (rate >90 bpm) was found to be strongly associated with an increased risk of hospitalisation and adverse events (hazard ratio [HR]: 5.9, p<0.001).68
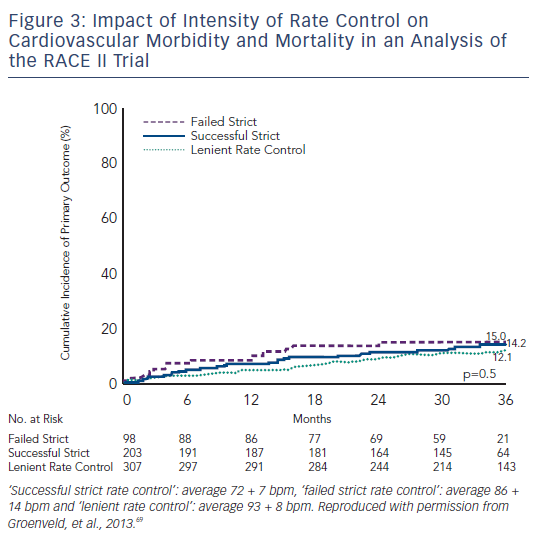
In a recently published analysis of the RACE II trial, three subgroups of rate control (‘successful’ strict rate: average 72 ± 7 bpm, ‘failed’ strict rate: average 86 ± 14 bpm and lenient rate: average 93 ± 8 bpm) were compared for the composite primary endpoints of cardiovascular morbidity and mortality, and no difference was observed in the three groups, and quality of life was also found to be comparable between groups.69 A strict rate control therapy could also prove to be time-consuming, necessitating more outpatient visits and more combinations and higher dosages of rate control drugs (see Figure 3).19,20,69,70
The interpretation of data from RCTs should, however, be individualised to patients in clinical practice; optimal heart rate control may vary on the basis of symptom burden and presence of co-morbidities, and patients with heart failure may represent a special group for whom strict rate control may be particularly beneficial. Additionally, it is possible that the impact of the adequacy of rate control may take many years to manifest and may not have been fully captured in trials such as RACE II, which followed patients for up to three years. If a patient remains symptomatic despite a lenient rate control or if there is a higher predisposition to develop cardiomyopathy, then lower heart rate targets may be indicated.
Methods of Achieving Rate Control in Patients with Atrial Fibrillation
In the AFFIRM trial, beta (β)-blockers (with or without digoxin) were found to have an overall success rate of approximately 70 % for achieving rate control when used either alone or in combination with digoxin, which was superior to the 54 % success rate of calcium channel antagonists (with or without digoxin).19,70 Recently published results from the RATe control in Atrial Fibrillation (RATAF) study contradicted the findings of the AFFIRM trial in regard to the comparative efficacy of β-blockers versus calcium blockers for rate control. RATAF trial was a prospective cross-over study comparing four drug regimens (diltiazem 360 milligrams (mg)/day, verapamil 240 mg/day, metoprolol 100 mg/day and carvedilol 25 mg/day) to reduce ventricular rate in patients with permanent AF. RATAF showed that calcium blockers (particularly diltiazem at 360 mg/day) were superior to the other drugs tested for achieving 24-hour heart rate control and also fared better in terms of control of symptom frequency and severity.71 Current guidelines recommend use of either a β-blocker or non-dihydropyridine calcium channel antagonist as a first-line agent for rate control in patients with AF.2 For many patients, either class of drugs will be acceptable, although for patients with certain co-morbidities, one drug class will clearly be preferred over the other (β-blockers preferred for heart failure patients, for example, and calcium blockers preferred for patients with bronchospasm).
Digoxin as a single agent has not been found to be as effective as β-blockers or calcium blockers in controlling ventricular rates in patients with AF; its efficacy is further reduced in the states of high sympathetic tone, which could be a precipitating factor in certain forms of paroxysmal AF or during exercise. The use of digoxin as a single agent should be reserved for sedentary patients or those with left ventricular systolic dysfunction who can be adequately rate controlled with digoxin alone (although systolic dysfunction may indicate potential benefit from β-blockade).2,72–74 Concern about digoxin use for rate control has been increased recently by newly published data from the AFFIRM trial, which revealed a significant increase in all-cause mortality with the use of digoxin. In a subgroup analysis of patients without congestive heart failure (CHF), digoxin was found to be associated with a 37 % increase in mortality. It should be noted, however, that this was not based on randomisation to digoxin or placebo, and there could be unidentified differences in the patients who received digoxin in the AFFIRM trial contributing to this outcome.75 Digoxin can be considered in combination with another rate control agent in patients with AF and left ventricular systolic dysfunction and patients who fail to achieve adequate rate control with a single agent.2,73,74
Intravenous (IV) amiodarone may be particularly effective as a rate control medication in critically ill patients who develop uncontrolled and haemodynamically compromising high ventricular rates during AF despite the use of conventional agents or patients who are intolerant to the conventional rate controlling agents.76 The rate controlling effects of amiodarone could be potentially attributed to its calcium channel blocking as well to its antiadrenergic properties.77
Ideally the selection of a drug for rate control should be made on the basis of results of RCTs, patient preference and presence of co-morbidities. It should also be important to bear in mind that these medications are not mutually exclusive; although a significant number of patients respond to monotherapy, a combination of rate control agents can be considered in patients who fail to achieve an adequate rate control with a single agent. Various interactions among these drugs, and the effect of electrolyte imbalance and fluid status of the patient should also be kept in mind while prescribing combinations of these medications, and careful attention should be paid to patients given both β-blockers and calcium blockers, as severe bradycardia can ensue.
Conclusions and Recommendations
Atrial fibrillation remains a common public health problem responsible for increasing morbidity, mortality and healthcare costs. Despite advancements in the field of rhythm control and availability of newer antiarrhythmics, rate control continues to be a preferred and therapeutically convenient option in patients who are older, minimally symptomatic and those who might not tolerate the adverse effects of currently available antiarrhythmic drugs. Catheter ablation is increasingly being used as an effective rhythm control strategy for many patients with drug-refractory AF, but there are not yet definitive data available in regard to hard endpoints such as mortality and stroke, which would allow ablation to be recommended as first-line therapy for the majority of patients with AF. Rhythm control may be preferred in younger patients, those who might benefit from prevention of the progression of AF and patients who remain symptomatic despite an optimal rate control. Management decisions can be guided by the available literature, physician preference and the symptom burden of the patients. Currently available data (see Table 1) supports the use of lenient rate control as a front-line strategy over strict rate control for most patients treated with rate control, although heart failure patients may benefit from more aggressive rate control targets.







