The monitoring of atrial fibrillation (AF) can be performed using a great variety of strategies and tools. Strategies range from monitoring only symptomatic AF (e.g., post-catheter ablation with or without surface electrocardiogram [ECG] documentation) to continuously monitoring heart rhythm using implantable pacemakers, implantable cardioverter defibrillators (ICDs) or subcutaneous implantable cardiac monitors (ICMs).1,2 With implanted devices, AF monitoring can be performed by interrogation during clinic visits as well as remotely.
Recent studies using different monitoring tools, ranging from transtelephonic monitoring to pacemakers, have indicated that the correlation between AF episodes and patient symptoms is very poor: many AF episodes are asymptomatic, whereas many AF-like symptoms are not related to AF episodes.3–13
The relevance of AF monitoring in clinical practice includes evaluation of the efficacy of a rhythm control strategy such as AF ablation and identification of the need for oral anticoagulation.14,15 In ICD patients, AF occurrence may increase the risk of inappropriate shocks and should therefore prompt careful evaluation of concomitant drug therapy and device programming. In cardiac resynchronisation therapy (CRT) patients, AF with fast ventricular response may limit the efficacy of cardiac resynchronisation and AF ablation or atrioventricular (AV) node ablation may be indicated.16
Current Status of Monitoring Tools
There are a variety of monitoring tools available to detect and record occurrences of AF. In clinical practice, because of the rather poor sensitivity of single standard ECG for paroxysmal AF, 24-hour ECG recording (Holter) is often used, allowing the detection of previously unrecognised AF.17–19
The extension of the ECG recording time from 24 to 72 hours increases the prevalence of paroxysmal AF after stroke from 1.2 to 6.1 %, suggesting that the probability of detecting AF increases with the duration of heart rhythm monitoring.20–22 External loop recorders (ELRs) are used to monitor the heart rhythm for one week or longer in an ambulatory patient. ELRs perform an event-triggered (e.g., onset of AF) or patient-triggered (e.g., onset of palpitations) sampling of the ECG during the period of use, with a limited storage capacity – for example 20 minutes.22–24 Other implantable devices capable of intracardiac atrial electrogram recording, such as dual-chamber pacemakers and defibrillators, can detect AF more appropriately due to their continuous monitoring capability.25 It was demonstrated that, by minimising the risk of artefact detection caused by myo-potentials or other sources of electrical interference, the detection of atrial tachyarrhythmia (AT) episodes lasting at least five minutes is well correlated with a proven diagnosis of AF.26 Several clinical studies have been successfully conducted using the diagnostic features of implanted dual chamber pacemakers or ICDs to evaluate AF burden and assess different therapeutic strategies.27–30
Another option for AF monitoring consists of subcutaneous ICMs, which, unlike pacemakers and ICDs, cannot sense endocardial atrial activity, but rely on the analysis of consecutive RR intervals to diagnose AF. The irregularity of the RR interval is now a proven parameter for AF detection, based on mathematical tools such as the Lorenz plot. In a Lorenz plot, each RR interval is plotted against the previous value of the RR interval, and this can be displayed graphically and used to discriminate between AF and sinus rhythm. A validation study, the Reveal XT performance trial (XPECT), showed that an ICM equipped with an algorithm for AF detection can accurately measure AF burden (98.5 %) and is very sensitive (96.4 %) to identify patients with AF, independent of symptoms. The device automatically stores a two-minute ECG strip for visual inspection and confirmation by the physician.31,32
Intermittent Versus Continuous Atrial Fibrillation Monitoring and its Clinical Relevance for Rhythm Control Strategies
In some areas of clinical practice, it is not essential to have a continuous rhythm monitoring established. For example, in patients with oral anticoagulation and a rhythm control strategy, it is not of major importance whether they may have a few silent recurrences or not. Therapy in those patients is guided by symptomatic recurrences on the one hand, and information about basic AF characterisation in terms of a paroxysmal or persistent AF pattern on the other hand. Knowing the exact AF burden in addition to symptomatic recurrences is an interesting add-on but not crucial. In contrast, in some other patient cohorts, continuous and ‘gapless’ information about the rhythm status is mandatory. Patients who have an uncertain correlation of symptoms (possibly triggering significant therapy) to the underlying rhythm are objects for continuous monitoring. In addition, checking for silent AF in high-risk populations, possibly leading to the initiation of oral anticoagulation, is a wide area for future applications of continuous monitoring devices (see next section). In addition, in order to measure efficacy of approaches (e.g., rhythm control) and therapeutic techniques, it is more and more accepted that we need continuous rhythm information, because spotlight ECGs are missing a major part of the AF activity which may lead to a wrong evaluation of therapy efficacy.
Continuous Versus Intermittent Rhythm Monitoring in Transvenous Catheter Ablation of Atrial Fibrillation
The relevance of increasing the Holter observation length from 24 hours to seven days has been demonstrated by Kottkamp et al.33 One hundred patients underwent 24-hour and seven-day Holter monitoring post-pulmonary vein ablation for paroxysmal AF. At 12 months, ablation success rate was 88 % when using the 24-hour Holter data, but only 74 % as indicated by the seven-day Holter. One might argue that this gap in capturing recurrences outside a Holter registration interval could be closed if an external event recorder would be used. However, Klemm and co-workers demonstrated that, in 80 post-ablation patients using transtelephonic ECG recordings (minimum one ECG per day and in case of symptoms suggesting AF), during 54 % of transmitted ECGs demonstrating AF patients were asymptomatic.8 In 11 % of all tracings, the patients indicated symptoms but demonstrated stable sinus rhythm on the tracing. In line with this finding, it was demonstrated that, outside a blanking period of three months, the ablation success in patients with only symptomatic but ECG-documented recurrences was around 70 %, whereas, counting all ECG-documented AF recurrences using a tele-ECG concept, the percentage of patients with no AF recurrences went down to almost 40 %.35 It was then Pokushalov and co-workers who reported their wide and routine use of ICMs to perform long-term continuous AF detection in their AF ablation patients. Furthermore, they differentiated between sudden-onset and triggered-onset AF recurrence to individualise the therapeutical regimens for AF recurrences after the first catheter ablation.35,36 An AF burden of >4.5 % measured with continuous rhythm monitoring during the two months post-ablation blanking period was a powerful predictor of ablation failure. This information and correlation could trigger early re-intervention in those patients, shortening the period of time until stable sinus rhythm is reached.2
Continuous Versus Intermittent Rhythm Monitoring in Surgical Ablation of Atrial Fibrillation
Recent data indicate that, similar to catheter ablation, with surgical ablation, there is a significant proportion of patients with silent and intermittent AF recurrences, whereas a significant number of symptomatic AF episodes are not related to AF. Ip and co-workers report that in about 45 AF patients, who underwent video-assisted epicardial ablation and ICM implantation, as much as 46 % of the AF recurrences were asymptomatic, whereas only 66 % of the symptomatic episodes were AF-related.37
Bogachev-Prokophiev et al. reported ablation results for AF concomitant with mitral valve surgery after one year of continuous monitoring.38 Forty-seven patients with mitral valve disease and long-standing persistent AF underwent a left atrial maze procedure with bipolar radiofrequency ablation and valve surgery. The follow-up data recorded by an ICM were analysed after three, six and 12 months. On discharge, 40 (85.1 %) patients were in stable sinus rhythm as documented by in-clinic ECG; 4 (8.5 %) were in pacemaker rhythm; and 3 (6.4 %) were in AF. One (2.1 %) patient died after seven months. Upon 12-month follow-up examination, 30 (65.2 %) patients had an AF burden <0.5 % and were classified as responders. Three (6.5 %) of the 16 non-responders had atrial flutter and 13 (27.7 %) had documented AF recurrences with an AF burden >0.5 %. Two (4.3 %) patients with AF recurrences were completely asymptomatic. Among the symptomatic events stored by the patients, only 27.6 % were confirmed as genuine AF recurrences according to the concomitant ECG recorded by the ICM.38 Again, the results in these surgical AF patients demonstrate the importance of continuous rhythm monitoring, not only to verify the true amount of successful ablation therapy, but also to identify asymptomatic recurrences and to better discriminate between AF- and non-AF-related symptoms.
Atrial Fibrillation Detection and its Relevance to Oral Anticoagulation Therapy for Stroke Prophylaxis
AF has been associated with a fivefold increase in the risk of ischaemic stroke.39 When the risk of stroke is consistent, oral anticoagulation (OAC), with vitamin K antagonists or novel anticoagulants, is required for effective prevention of thromboembolic events. However, underutilisation of OAC therapy with warfarin has been shown to occur in ‘real world’ clinical practice in patients at moderate to high thromboembolic risk.40,41 Current guidelines for stroke prevention in AF39 do not differentiate between patients with symptomatic or asymptomatic AF, or between paroxysmal or persistent AF. The vast majority of AF episodes are asymptomatic, including many episodes of clinically significant duration.42 Implanted devices can provide continuous information on atrial rhythm as the basis for more accurate and extended diagnostic capabilities. On the basis of data provided by device diagnostics, the term ‘AF burden’ has been proposed as the total amount of time spent in AF per monitored time period and a duration of >5 minutes has been associated with stroke and death.43–46
In summary, a series of studies performed in pacemaker or ICD patients indicate that longer times spent in AF are associated with slightly higher stroke risk, and that this increased risk is additive to well known stroke risk factors.43–46 A recent study performed on pacemaker patients assessed non only the CHADS2 score but also the CHA2DS2-VASc score, and found that implementation of device data on AF presence/duration/burden has the potential to contribute to improved clinical risk stratification.47
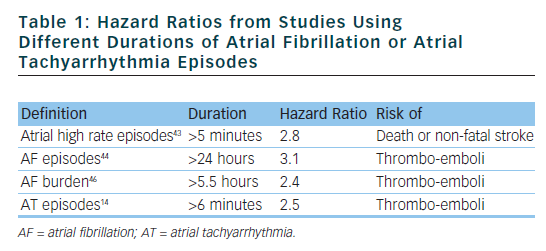
Recently, the ASSERT (Asymptomatic atrial fibrillation and stroke evaluation in pacemaker patients and the atrial fibrillation reduction atrial pacing trial) study included 2,580 patients aged 65 years or older, with hypertension and no history of AF, in whom a pacemaker or ICD had recently been implanted.14 Subclinical ATs (episodes of atrial rate >190 beats per minute for more than 6 minutes) occurred in 10 % of patients in the first three months and were associated with an increased risk of clinical AF (hazard ratio [HR] 5.56) and of ischaemic stroke or systemic embolism (HR 2.49). The conclusions of ASSERT were that subclinical ATs occur frequently in patients with an implanted device and are associated with a significantly increased risk of ischaemic stroke or systemic embolism. Table 1 shows the HRs from studies using different durations of AT or AF episodes.
The open question is: how often is the detailed and precise information on AF burden provided by devices (pacemakers, ICDs or ICMs) actually translated into clinical decision-making for the optimisation of thromboembolic prophylaxis? The ANGELS of AF (Anticoagulation use evaluation and life threatening events sentinels) project was a medical care programme aiming to improve OAC use as thromboprophylaxis, with the use of the information from the ICD AF diagnostics.48 The results demonstrate that a medical care programme may improve OAC implementation in standard medical practice by supplying accurate and continuous information about stroke risk factors, AF occurrence and burden.
In detail, 50 Italian cardiology clinics followed 3,438 ICD patients. In a subgroup of 15 centres (the ANGELS of AF centres), cardiologists attending follow-up visits were supplied with specific reports describing stroke risk factors and risk scores, including CHADS2, AF occurrence and duration, and current antithrombotic therapy for patients with AF (especially those with CHADS2 score >0 and without OAC therapy). The remaining centres represented a control group of patients as a comparison for OAC use. In the 15 ANGELS of AF centres, 36 % patients had AF and 29 % were not taking OAC. Appropriate OAC therapy was prescribed in 10 % of patients after evaluation of Angels of AF reports. The percentage of patients on OAC therapy, as indicated by guidelines, increased during follow-up from 46.1 % at baseline to 69.4 % at stroke risk evaluation phase, and up to 72.6 % at the end of the observation period. In control centres, corresponding figures were 46.9 % at baseline and 56.8 % at the end of the observation period (p<0.001 versus Angels AF group).
Since disparities in the use of guideline-recommended and evidence-based therapies for AF in inpatient and outpatient care settings are well documented, it is well known that a major underutilisation of OAC therapy in particular has been observed in numerous studies evaluating clinical practice.40 As a result, many AF patients may suffer embolic events, such as stroke or transient ischaemic attacks, which might have been prevented by appropriate detection through continuous monitoring of AF. The ANGELS of AF project demonstrates the possibility to improve OAC use in accordance with available guidelines for stroke risk reduction in AF by supplying attending physicians with reports about patients risk factors and AF information from continuous device monitoring.
A more focused device monitoring will be tested in CRYSTAL AF (Study of continuous cardiac monitoring to assess atrial fibrillation after cryptogenic stroke), a randomised prospective trial to evaluate a novel approach aimed at comparing standard arrhythmia monitoring (control arm) with implantation of a subcutaneous ICM (Reveal® XT, Medtronic, Inc., Minneapolis, Minnesota, US) (continuous monitoring arm) to detect AF in cryptogenic stroke patients.
Clinical Consequences of Atrial Fibrillation Monitoring in Implantable Cardioverter Defibrillator Therapy and Cardiac Resynchronisation Therapy
Avoidance of high-energy shocks remains an important goal in the management of patients with ICDs.16 Patients with inappropriate shocks more commonly have a history of AF, smoking and/or diastolic hypertension, and are more likely to also have had a prior appropriate ICD shock. Other investigators have found that a prior history of AF and appropriate therapy were predictors of inappropriate shocks.49 Because AF was the most common inappropriate shock mechanism, its association as a predictive factor is expected. Smoking was recently found to increase the incidence of both appropriate and inappropriate shocks in the MADIT (Multicenter automatic defibrillator implantation trial) II study, possibly because of a myriad of adverse consequences such as sympathetic stimulation, increased platelet reactivity, vasoconstriction, endothelial dysfunction and tachycardia.50 Hypertension is a potent risk factor for AF and may act in this way to promote the likelihood of an inappropriate shock. The link between appropriate and inappropriate therapy likely stems from a combination of several factors:
- ventricular arrhythmia or its treatment (antitachycardia pacing techniques or shock) provoking AF;51,52
- inappropriate therapy causing ventricular tachycardia (VT); that is, pro-arrhythmia;53
- a common factor or factors predisposing to both VT/ventricular fibrillation and AF or supraventricular tachycardia; or
- incorrect categorisation of some appropriate episodes in a given patient as inappropriate or vice versa.54
Numerous trials have identified a rate of inappropriate shocks between 20 % and 30 %, most commonly due to rapidly conducted AF. It was demonstrated that poor rate control often occurs days and even months before shocks, and therefore continuous monitoring along with the use of patient or wireless alerts may be warranted. The use of continuous monitoring along with automated alerts to provide early notification of AF with a fast ventricular rate may help clinicians take action early in order to prevent future shocks.16
There has been increasing recognition of the epidemics of heart failure (HF) and AF, conditions often present in the same patient. Initially validated in populations in normal sinus rhythm, CRT now has an established role in the treatment of HF patients with left ventricular (LV) dysfunction and intraventricular conduction delay. The incidence of new AF in patients who have been implanted with a CRT device is substantial, exceeding one-fourth of patients.
It is clear that CRT is not beneficial if biventricular (BiV) pacing does not reliably occur. This is because the physiological impact of CRT is postulated to occur through synchronising ventricular contraction, leading to improvement in LV filling and pumping efficiency and reduction in the extent of functional mitral regurgitation. In patients with HF and AF, AV synchrony does not exist; thus, any clinical benefit is predicated on BiV synchronisation. However, in patients with AF, erratic electric activity of the atria occasionally will penetrate the AV node and override, interrupt or disrupt BiV capture.55,56 This leads to fusion or pseudo-fusion beats and suboptimal CRT delivery, particularly in situations of increased myocardial demand, as occurs from increased adrenergic tone during stress or exercise. Perhaps even more problematic than the lack of BiV capture is that the percentage of BiV pacing recorded by internal CRT device counters is often artificially inflated during AF, due to erroneous counting of fusion and pseudo-fusion complexes.57 Although a clinician might believe that adequate delivery of CRT has occurred because of an apparently high percentage of BiV pacing, a patient with AF may not have received the full benefit of synchronised BiV capture.58
To rely on device counters alone can be misleading and to review data from the device diagnostics or to optimise the AF detection is instrumental to ensure that pseudo-fused and fused beats are not being miscounted as those associated with BiV capture.
Santini et al. have monitored AF in 1,193 HF patients with a CRT-D (CRT defibrillator) and found that 30 % had AF episodes lasting longer than 10 minutes.59 Compared with the group that had only sinus rhythm, the group with AF episodes had a 2.16 times higher risk of mortality or HF hospitalisation. The authors concluded that AF detection in CRT-D patients is important to identify patients at risk of cardiac deterioration or patients with suboptimal rate or rhythm control.
Conclusion
We have reviewed the major areas of clinical indication for AF monitoring. For outcome measurements in AF treatment trials comparing rhythm versus rate control or different rhythms strategies continuos AF monitoring is gaining more and more importance. It is quite obvious that intermittent ECG documentation only captures a minority of the true AF burden. Whereas, in the pure clinical arena especially in the field of rhythm control correlation of symptoms and underlying rhythm is still more relevant than capturing different levels of AF burden.
The method for AF monitoring which should be used has to be tailored to the patients situation; implantable long term ECG monitors offer a lot of advantages but require the willingness of an invasive approach.
Detecting AF in patients with risk factors should be performed wherever it is possible, especially when we have implanted cardiac rhythm devices. We know that even short lasting AF episodes with a low AF burden already induce a significant risk increase, therefore an electrogram-documented and -verified device-based AF detection should trigger the process of starting OAC as recommended for every patient with risk factors and surface ECG documented AF. In addition, device-based AF detection has importance for device therapy itself like device programming, e.g. to prevent inadequate shock therapy from rapidly conducted AF in defibrillator patients.
One step further; searching for AF in high risk populations with so far not-documented AF – like the cryptogenic stroke population – is subject of scientific studies and has the potential for enlarging the indication for AF monitoring significantly.
Termination of OAC in patients with risk factors and AF is not recommended; however in selected cases with manifest bleeding or an excessive risk for bleeding it may be necessary to execute a bail-out concept with either left atrial occluder implantation or rhythm control with continuous monitoring and triggered OAC in case of arrhythmia recurrence.