Sir James Mackenzie, famous for describing the first mechanistic insights into AF in 1902 using his polygraph, also reported that AF was present in 80–90% of patients who had congestive heart failure (HF) in 1920.1 Today, the conditions are the two ‘epidemics’ of cardiovascular disease.2 They are dominating cardiovascular care and, with increasing longevity, they will become more prevalent and place an even greater burden upon healthcare resources over the coming decades.3, 4 The conditions are inextricably linked in a vicious cycle, with HF promoting the development of AF and vice versa. In addition, each increases the morbidity and mortality associated with the other.5
Despite good progress in the management of AF-related symptoms, there are limited data to compare the benefits of different treatments and international guidelines advocate multiple therapeutic options.6 Traditionally, AF rhythm control involves a combination of antiarrhythmic medical therapy and direct current cardioversion (DCCV). Partly because of the inefficacy of these therapies the ‘rate versus rhythm’ debate has been intense in the aftermath of trials showing that, compared to a rate control strategy, a rhythm control strategy does not reduce mortality or morbidity and is more costly and inconvenient.7, 8
More recently, multiple studies have reported improvements in ‘soft’ end points with catheter ablation while two trials – Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA; NCT00911508) and Catheter Ablation vs Standard Conventional Treatment in Patients With LV Dysfunction and AF (CASTLE-AF) – have reignited the debate as to whether modern rhythm control therapy can improve prognosis in patients with AF.
This paper is a state-of-the-art review analysing world literature accessed via detailed literature searches utilising PubMed, Web of Science and Scopus to establish the connection between AF and HF in detail and to determine the impact of AF rhythm control on patients with coexisting HF.
Direct Current Cardioversion for AF and Left Ventricular Performance
In 1962, Lown described electrical cardioversion of AF.9 He later won the Nobel Prize for his nuclear weapon non-proliferation work.10 Electrical cardioversion is indicated for patients with AF associated with significant symptoms or as part of a long-term rhythm control strategy. The efficacy and immediacy of DCCV in restoring SR provides valuable insight into the potential benefit of rhythm control on cardiac performance.
Kieny et al. demonstrated that, after successful cardioversion in persistent AF patients with dilated cardiomyopathy, left ventricular ejection fraction (LVEF) improved from 32.1% ±€5.3% to 52.9 ±€9.7%; p<0.001.11 Wall et al. demonstrated an improvement in LVEF of 14.2% in patients with impaired LV function following successful cardioversion (n=108; 95% CI [11.0%–17.4%]; p<0.0001). Furthermore, the benefit was more significant the lower the LVEF. The subgroup analysis of moderately reduced ejection fraction (HFmrEF) showed a mean improvement of 4.24% (n=50; 95% CI [0.3–8.2%]; p=0.03) and the subgroup analysis of reduced ejection fraction (HFrEF) showed a mean improvement of 23.0% (n=58; 95% CI [19.4–26.6%]; p<0.0001).
DCCV successfully restores SR in the majority of patients who undergo the procedure with quoted success rates at the time of the procedure of the order of 85%.12 However, it is widely accepted that DCCV has limited long-term success rate with only 30–40% of patients remaining in SR at the end of 1 year.13 Restoration of SR with DCCV can improve AF-related symptoms, LVEF, exercise capacity and HF symptoms.14,15
Given the greater efficacy of AF ablation and antiarrhythmic drug (AAD) therapy in maintaining SR, it is logical to hypothesise a more substantial role for these interventions in patients with coexisting HF and AF. Despite this, the only indication in international guidelines for catheter and surgical AF ablation (including concomitant open and closed procedures and standalone) remains symptom relief.16
Antiarrhythmic Medication for AF in Heart Failure
Two landmark studies, each with >1,000 patients, have assessed the efficacy of pharmacological rhythm control in patients with concomitant AF and HF (AF-CHF) with HFrEF.17 In the Danish Investigators of Arrhythmia and Mortality on Dofetilide in Congestive Heart Failure (DIAMOND-CHF) trial, 1,518 patients were randomised to receive either dofetilide (n=762) or placebo (n=758). At the conclusion of the trial (12 months follow-up), 65% of patients in the dofetilide arm were in SR versus 30% of patients in the placebo arm. There was no difference in mortality between the two groups, but the dofetilide arm had lower rates of HF hospitalisation than the placebo group.18
In the AF-CHF trial, there was no difference in cardiovascular death when comparing a rate versus rhythm-control strategy with antiarrhythmic medications in 1,376 patients with AF and HFrEF and New York Heart Association (NYHA) classes II–IV (HR 1.06; 95% CI [0.86–1.30]; p=0.59), with similar findings for all-cause mortality and worsening HF.19
A possible explanation for these neutral outcomes is the difficulty in achieving and maintaining SR in patients with HF. In the rhythm control arm of AF-CHF, although 82% or participants were taking amiodarone, 58% had at least one episode of AF during the trial.19 In addition, the potential benefit of SR maintenance with respect to mortality may have been neutralised by harmful effects of AADs.17
Benefits of Rate Control for AF in Heart Failure
A poor rate control resulting in fast ventricular response has been suspected as one of the major determinants of HF in AF patients. Impaired cardiac function can be reversed after restoration of SR and good ventricular rate control achieved as well by using either antiarrhythmic drugs or by atrioventricular (AV) node ablation and pacemaker implantation.2
While the benefit of cardiac resynchronisation therapy (CRT) is established in symptomatic HF patients in SR with LVEF ≤35% and QRS duration of ≥120 ms, its role in patients with coexistent HF and AF is less well defined.20,21 CRT with AV node ablation provides robust rate control and improved ventricular synchrony in AF and requires attention. Three studies have evaluated the impact of AV node ablation on LVEF in 346 CRT-AF patients.22–24 The mean increase in LVEF was 10.3% (95% CI [6.4%–14.2%]) in patients receiving a CRT device combined with AV node ablation. These data suggest an important role for rate control of AF in improving outcomes in HF patients.
Catheter Ablation for AF in Heart Failure
The first data on the impact of curative catheter ablation for AF in HF patients was reported by Hsu et al. in 2004.25 The authors demonstrated that LVEF significantly increased after AF ablation with the greatest improvement within the first 3 months after the procedure. Interestingly, LVEF increased in most of the patients irrespective of whether ventricular rates were poor or well-controlled before ablation, indicating the existence of other factors than a fast ventricular rate for the development of AF-CHF.
In the Comparison of Pulmonary Vein Isolation Versus AV Nodal Ablation With Biventricular Pacing for Patients With Atrial Fibrillation With Congestive Heart Failure (PABA CHF; NCT00599976) study, 41 patients with drug-resistant AF were randomly assigned to pulmonary vein isolation (PVI) and 40 patients to undergo AV node ablation combined with biventricular pacing.26 At 6 months, patients who had undergone PVI had a higher LVEF than those who had received AV node ablation and biventricular pacing (35% versus 28%; p<0.011). Patients undergoing the rhythm control procedure also had better 6-minute walk distance (340 m versus 297 m; p<0.001) than those in the ‘ablate and pace’ strategy. In patients undergoing PVI, 71% remained in SR at 6 months. AV node ablation with biventricular pacing is a robust form of the rate-control strategy and of rate regularisation. PABA CHF showed that PVI, compared to the best possible rate-control and rate-regularisation strategy, provides superior morphological and functional improvements. Potential explanations for LVEF improvement might be the improvement of atrial contractility, maintenance of atrioventricular synchrony, as well as the prevention of high ventricular rates.27
Importance of Sinus Rhythm
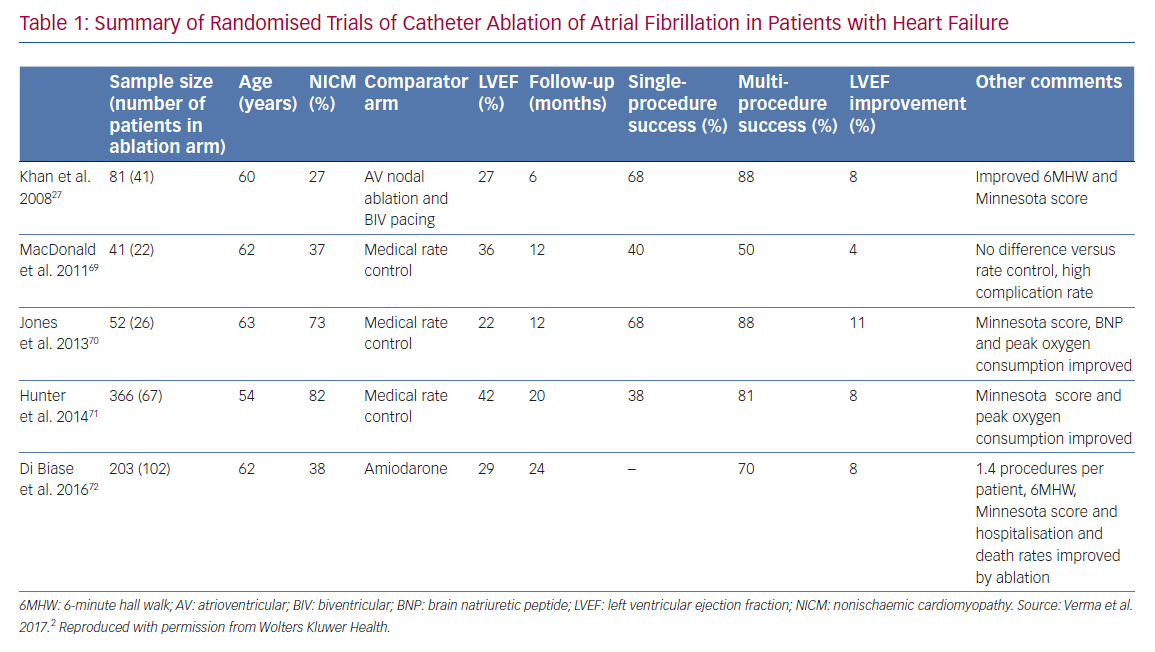
A number of recent trials have suggested that SR following AF ablation is associated with improved outcomes in patients with AF.28 Substantial data demonstrate that restoration of SR leads to an improvement in LVEF in AF patients (Table 1).29 Regardless of aetiology, LV systolic dysfunction and HF are associated with a higher risk of death.30 The majority of AF ablation trials use freedom from AF and restoration of SR as their primary endpoints, with procedural success rates of 50–60% after a single procedure and 80–85% after repeat procedures.31 Therefore, it is logical to postulate that, in restoring SR, a successful AF ablation may not only improve LVEF but also reduce the excess mortality associated with concomitant HF.
In studies of catheter ablation of AF, restoration of SR is associated with significant improvements in LVEF, with an 11% increase on average.32 In addition, patients with AF and HF who spend a higher proportion of time in SR experience less severe functional impairment (NYHA class III symptoms in 27 versus 35%; p<0.0001).33
Myocardial Fibrosis in AF and Heart Failure
Atrial fibrosis leads to structural and functional impairment of the left atrium and persistence of AF, and is associated with the development of AF-HF.34,35 Mild pre-ablation left atrial structural remodelling by delayed enhancement MRI (DEMRI) predicts favourable structural and functional reverse remodelling and long-term success after catheter ablation of AF, irrespective of the paroxysmal or persistent nature of AF.34
Despite extensive research addressing the interplay between changes in the atria and AF, relatively few studies provide histological evaluation of the ventricle in patients with AF.36 However, it appears to have a crucial role in the AF-CHF interaction. Ventricular fibrosis may occur secondary to AF as a consequence of rapid ventricular rates, the irregularity of ventricular contraction or activation of the renin–angiotensin–aldosterone system.35,37,38 Myocardial interstitial fibrosis contributes to left ventricular dysfunction leading to the development of HF.39 Successful catheter ablation has been shown to result in reverse remodelling and a regression of diffuse fibrosis in AF-mediated cardiomyopathy providing the pathophysiological explanation for the benefit of ablation in AF-CHF patients.40
Cardiac MRI offers noninvasive assessment of atrial injury and recovery of active atrial function following AF ablation because of its ability to visualise all segments of the atrial wall during the cardiac cycle.41 Catheter ablation can be associated with sustained atrial dysfunction owing to to ablation-related scarring. Previous studies have demonstrated that the difference between electroanatomic mapping (EAM) ablated area and LGE-MRI scar area was associated with higher AF recurrence after ablation.42 Despite the aforementioned benefits of catheter ablation in AF-CHF, repeat ablation could be associated with more ablation-related scarring and worse outcomes.43,44 This suggests timely treatment of arrhythmia-mediated cardiomyopathy may minimise irreversible ventricular remodelling if SR is restored and multiple AF ablation procedures should be avoided.
Cardiopulmonary Exercise Testing in AF and Sinus Rhythm
Cardiopulmonary exercise testing (CPET) is an important tool to evaluate exercise capacity and predict outcomes in patients with HF.45 It provides an assessment of the integrative exercise responses involving the pulmonary, cardiovascular and skeletal muscle systems, which are not adequately reflected through the measurement of individual organ system function.45 Peak oxygen uptake (VO2 peak) is an important, reproducible facet of exercise performance and has been shown to have high prognostic value in cardiac patients and healthy individuals. VO2 peak is determined by cellular oxygen demand and equates to the maximal rate of oxygen transport. Significant increases in VO2 peak in SR have been demonstrated on CPET in patients who have undergone AF ablation.46 These findings imply that an improvement in haemodynamics in SR improves the rate of oxygen transport and, ultimately, this has the potential to improve prognosis. In addition, VO2 peak is a strong prognostic indicator in chronic HF and is a criterion variable for consideration of cardiac transplantation in such patients.47,48 Among patients with chronic systolic HF, even a modest increase in peak VO2 peak over 3 months has been associated with more favourable outcomes, highlighting the importance of CPET as an investigative tool; it also provides an insight into the favourable haemodynamic effects of restoring SR with an ablation procedure.49
Sleep Studies and Rhythm Control
Another condition strongly associated with AF is sleep-disordered breathing (SDB). Perhaps the most straightforward explanation for the association is that patients with AF and SDB share a number of risk factors and comorbidities, including age, male sex, hypertension, HF and coronary artery disease.
More evidence is emerging of a true physiological connection.50,51 Patients with obstructive sleep apnoea (OSA) have >30% greater risk of AF recurrence after catheter ablation than those without.52–54 However, the efficacy of catheter ablation for AF is similar in patients without obstructive sleep apnoea and those with this condition who are on continuous positive airway pressure treatment.55,56
In an animal model, obesity and acute obstructive apnoea have been shown to interact to promote AF.57 OSA is associated with repetitive forced inspiration against a closed airway which can result in negative intrathoracic pressure leading to an increase in cardiac afterload, larger atrial size and higher wall stress, resulting in atrial remodelling, which predisposes patients to arrhythmia.58
Further recent studies have demonstrated a reduction in nocturnal respiratory events (apnoeas and hypopnoeas) and a reversal of sleep-disordered breathing with restoration of SR using both DCCV and AF ablation procedures at short-term follow-up.55,56 Improving haemodynamic status and cardiac function with restoration of SR could reduce fluid displacement from the lower limbs to the neck region of the body, a key mechanism in the pathogenesis of OSA.59–61 The hazard of mortality in sleep apnoea increases with apnoea severity, highlighting the potential importance of these findings and providing a further, different angle to hypotheses supporting a mortality benefit of SR in patients with AF.62
AF Ablation and Mortality in Heart Failure
A number of studies postulate that AF ablation can reduce mortality. CASTLE-AF is the only randomised clinical trial to date comparing catheter ablation and pharmacological therapy for patients with coexisting HF and AF that measures the ‘hard’ primary endpoints of death and hospitalisation for heart failure.63 Patients had symptomatic paroxysmal or persistent AF, LVEF ≤35%, NYHA class ≥2, with an ICD or CRT with defibrillator implanted. AF ablation was associated with a significantly lower rate of a composite of death and hospitalisation for HF than medical therapy.63 There was also a benefit in all-cause mortality alone, driven by a significantly lower rate of cardiovascular death in the ablation group.
Furthermore, catheter ablation reduced the AF burden, increased the distance walked in 6 minutes and improved the LVEF. On the basis of the data extracted from the memory of the implanted devices, 63.1% of the patients in the ablation group and 21.7% in the medical-therapy group (p<0.001) were in SR at the 60-month follow-up visit and had not had AF recur since the previous follow-up visit (typically at 48 months).63
Heart Rhythm Monitoring Following AF Ablation
Given the benefits of maintenance of SR following AF ablation described, accurate and complete heart rhythm monitoring is imperative. The HRS Expert Consensus Statement set guidelines for catheter ablation trials stating that, after the blanking period, success is defined as ‘freedom from AF, atrial flutter or tachycardia’ and discontinuation of antiarrhythmic medication, that patients should be followed for at least 12 months and, at minimum, should have a 24-hour Holter monitor at 3, 6, 12 and 24 months.16,64
The gold standard of heart rhythm monitoring is beat-to-beat monitoring with implanted devices.65 AF ablation studies employing beat-to-beat monitoring with implanted devices have determined ‘cure’ rates of only 29% for persistent AF, significantly lower than trials that used less stringent monitoring criteria.31, 66 Beat-to-beat monitoring will detect significantly more AF episodes because of the continuous monitoring capabilities of implanted devices. In the absence of large, prospective, randomised studies using beat-to-beat follow up, ablation success remains open to speculation. Beat-to-beat monitoring is particularly important if it is the restoration of SR that is associated with improvement in LV function and provides an argument for all catheter ablation studies to have significantly tighter cardiac monitoring, ideally with implanted devices allowing every heartbeat to be monitored.
Discussion
The main findings of our systematic review are that the pathophysiological benefits from AF ablation stem from successful restoration of SR and this is most likely to be achieved by early intervention. These benefits extend to reversed remodelling of the left cardiac chambers, an improvement in LVEF, an improvement in key, prognostic facets of exercise performance and a reduction in SDB. It is likely that these factors are the drivers for the reduced mortality observed with AF ablation in the recent CASTLE-AF study.
In addition, a large percentage of patients in the general population progress from a paroxysmal form of AF to a persistent or permanent form, limiting the likelihood of successful ablation, suggesting timely treatment of arrhythmia-mediated cardiomyopathy with ablation may minimise irreversible remodelling when SR is restored.
Finally, given the importance of restoration and maintenance of SR following ablation, we propose that AF ablation trials should use stringent heart rhythm monitoring, ideally with implanted devices, allowing monitoring of every heartbeat to document the true impact of ablation on heart rhythm. Long-term monitoring with an implanted device allows for determination of AF pattern, number of discrete episodes and AF burden, providing a wealth of information regarding a patient’s AF.
Recent evidence has suggested the importance of AF burden to cardiovascular and neurological outcomes, and the effect of lifestyle and risk factor modification on AF burden. AF burden is best defined as the proportion of time an individual is in AF during a monitoring period, expressed as a percentage, and continuous monitoring, ideally with an implanted device, is required to meet this definition.
A number of studies have reported improvement in ‘soft’ end points with catheter ablation of AF. However, they are not powered to demonstrate that mortality can be reduced by ablation. The CASTLE-AF trial substantiates these earlier reports that AF ablation is beneficial in patients with AF and HF. The study demonstrated that the use of ablation for AF in patients with HF is associated with a significantly lower composite of death and HF hospitalisation than medical therapy. The results from CASTLE-AF are of significant interest and support a role for AF ablation in such patients. However, these results do not support offering AF ablation to all patients with AF and HF. The inclusion criteria for the trial were strict, resulting in more than 3,000 patients being screened to identify 363 patients to take part in the trial. The quality of the rate control in the pharmacological group has not been published and, in the current review, we have demonstrated the importance of effective rate control in improving LV performance. The mortality benefits of ablation only appeared after 3 years into the trial, by which stage only 191 of the original trial patients were still being followed up. Finally, some subgroups did not benefit from ablation, such as those with an LVEF<25%.67 However, despite these issues, there is sufficient evidence to support early AF ablation in patients with symptomatic AF and HF, in addition to device therapy.
The Future
A significant limitation of all AF ablation studies is the lack of blinding with regard to randomisation and treatment. Randomised, double-blind, placebo-controlled studies are considered the gold standard of studies involving a medical intervention. Randomised clinical trials with inadequate blinding report enhanced placebo effects for intervention groups and nocebo effects for placebo groups.68
It is difficult to perform a truly blinded trial with a sham AF ablation procedure, but the lack of blinding could result in bias as to whether, for example, to admit a patient for worsening HF, how the patients are medically managed, how the patients report symptoms and so on. To date, no studies have included a satisfactory, ethically justifiable sham limb to compare with AF ablation. The advent of such a study design could advance our understanding to another level.
Clinical Perspective
- The pathophysiological benefits from AF ablation stem from successful restoration and maintenance of sinus rhythm and this is most likely to be achieved by early intervention.
- These benefits extend to reversed remodelling of the left cardiac chambers, an improvement in left ventricular ejection fraction, an improvement in key, prognostic facets of exercise performance and a reduction in sleep-disordered breathing.
- Timely treatment of arrhythmia-mediated cardiomyopathy with ablation may minimise irreversible remodelling when sinus rhythm is restored.
- Given the importance of restoration and maintenance of sinus rhythm following ablation, we propose that AF ablation trials should use stringent heart rhythm monitoring, ideally with implanted devices, allowing monitoring of every heartbeat to document the true impact of ablation on heart rhythm.