Atrial fibrillation (AF) is the most common clinical arrhythmia with a relevant socio-economic impact.1–3 Patients with atrial fibrillation are characterised by symptoms such as palpitations and shortness of breath; they have reduced exercise capacity and are subject to a higher risk of thromboembolic events. In spite of a relatively simple diagnosis, management of AF patients is difficult for the uncertainty of the optimal therapeutical strategy,4 for the limited efficacy and safety of almost all anti-arrhythmic drugs3,5 and for the presence of severe co-morbidities.3 More recent approaches based on targeting arrhythmia triggers such as radio-frequency ablation are actually utilised in a limited number of subjects for the complexity of the procedure and uncertainty of criteria for patients’ selection.6,7 The value of upstream therapy as an anti-arrhythmic tool has been recently questioned by its limited efficacy in controlled trials;8–10 nevertheless, most of AF patients are treated with angiotensin-converting-enzyme (ACE) inhibitors and beat-blockers for the concomitant presence of hypertension, coronary artery disease or heart failure.
In the last 20 years, a general consensus has been reached on the fact that AF cannot be viewed as a simple electrocardiographical alteration rather as a clinical disorder in which different factors acting as triggers or substrate modifiers may affect the clinical history and mortality of AF patients.1–3 A consistent feature is that AF patients have a greater mortality than patients with preserved sinus rhythm. However, it remains controversial if this is mainly due to a negative direct effect of the arrhythmia or to the co-morbidities and the increased thromboembolic risk associated with AF. Available epidemiological evidence11 indicates that a single electrocardiogram (ECG) recording of AF in a middle-aged woman increased her risk of cardiovascular events fivefold in the next two decades, whereas in men, the risk increased twofold. Most of this excess risk is related to age, heart failure and stroke.11 The critical value of the above factors has also been emphasised by the most recent international guidelines on AF,3 which have focused their suggestions on the identifications of predictors for an increased thromboembolic and haemorrhagic risk or for arrhythmia recurrences in the context of the different clinical conditions associated with this rhythm disturbance.
Thus the identification of factors affecting mortality and in particular, cardiac mortality in AF patients is mainly based on the characterisation of the concomitant cardiac and/or non-cardiac disease rather than on specific electrocardiographic features.
In the present article, some of the most important factors associated with increased mortality in AF patients will briefly discussed.
Ageing and Atrial Fibrillation
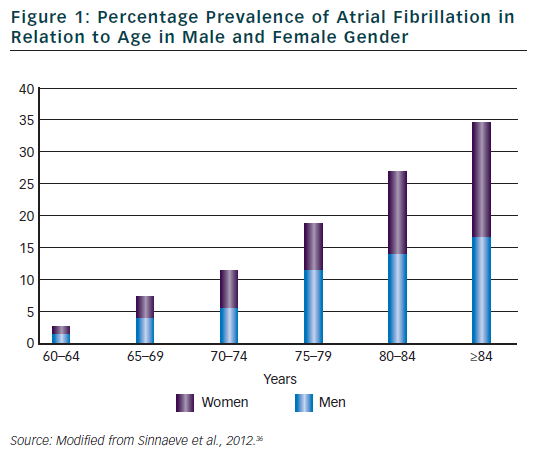
The association between ageing and AF has been for long recognised. Current figures12 indicate that in the year 2010, 53,364 centenarians were alive in US and that the incidence of people older than 65 and 84 years were, respectively, 13 and 1.8 %. This latter group presented the greatest percentage of proportional increase in comparison to what observed in the year 2000. The number of elderly subjects is therefore expected to display a further increase in the next decades. Being the prevalence of AF definitely correlated with age (see Figure 1), this will result not only in an overall increase in the total number of AF patients but will determine a further prevalence of a large subgroup of elderly subjects with multiple and severe co-morbidities, almost no indications for ablation strategies and highest risk for haemorrhagic and/or thromboembolic events. Regarding this latter point, it is worth noting that almost all of these patients, independently of the selected rhythm or rate control strategy, will have an indication for an oral anticoagulant therapy as suggested by current guidelines, being their CHADS2 score ≥2 for the presence of at least age and hypertension criteria (3). Data from the AFFIRM13 study have already confirmed the relevance of this factor. The rhythm control strategy was associated with a higher risk of death than the rate control strategy among older patients ( age >65 years) and most of strokes occurred after stopping anticoagulation therapy. As a consequence, rate control was considered possible and more effective that the rhythm control strategy in these high risk patients.14 A better appreciation of the potential pro-arrhythmic effects of all anti-arrhythmic drugs as well as of the individual thromboembolic risk and of the efficacy of new pharmacological and non-pharmacological therapies could however modify our approach for the selection of the most appropriate treatment of AF in the elderly patients.
Atrial Fibrillation, Heart Failure and Mortality
Heart failure (HF) and AF are frequently present in the same patient and concur to determine his prognosis1–3 HF by altering haemodynamic and neuro-humoral function as well as cellular and extracellular remodelling, may favour arrhythmogenesis. AF, by reducing stroke volume, inducing tachycardia cardiomyopathy and exposing patients to the potential toxicity of anti-arrhythmic drugs may also negatively affect mortality. Both conditions, in addition, are associated with an increased thromboembolic risk. When considering predictors of mortality in heart failure patients with AF, it was shown that age, chronic obstructive pulmonary disease, elevated serum creatinine on admission and increased QRS duration were independently associated with mortality. On the contrary, oral anticoagulant therapy determined a significant risk reduction. Of interest was the finding that in heart failure patients with AF, neither New York Heart Association (NYHA) functional class or left ventricular ejection fraction (LVEF) were independently associated with mortality thus suggesting that these parameters loose most of their predictive value when AF is present.15
Moreover, when the impact of AF was also evaluated in relation of early and long term prognosis in patients hospitalised for HF,16 it was shown that the arrhythmia was more frequently detected in elderly patients with modest reduction of LVEF. Mortality rates for AF patients were higher during hospitalisation and four year follow-up in comparison to patients in sinus rhythm. This difference was explained for the most part by co-morbidities including valvular heart disease, renal failure, diabetes and hypertension that were more frequently present in AF subjects.16
Cardiac Biomarkers and Increased Mortality in Atrial Fibrillation Patients
Being mortality associated with AF largely dependent upon co-morbidities and increased thromboembolic risk, identification of high risk patients could be facilitated by the measure of markers that reflect disease severity. A recent sub-study17 of the RE-LY trial18 has provided interesting results. At variance with patients in sinus rhythm where biomarkers have been extensively utilised to obtain diagnostic and prognostic information, so far no study has evaluated the prognostic role of Troponin I and N-terminal pro-b-type natriuretic peptide (NT-proBNP) in AF patients. Hijazi and colleagues17 measured baseline Troponin I and NT-proBNP levels in 6,179 patients enrolled in the RE-LY study. Patients were stratified on biomarker concentration quartile and outcomes were evaluated after adjustments for established cardiovascular risk factors and CHADS2 risk score. Rates of strokes were independently related to Troponin I levels and NT–proBNP quartile groups with a lower incidence at the lowest biomarker concentration. Vascular mortality was also independently related to biomarker levels. The results of the study showed that markers of myocardial cellular damage and increased myocardial wall tension were increased in almost one third of patients with non valvular AF and had important prognostic value: the degree of biomarker elevation was independently related to an increased risk of stroke or systemic embolism, mortality and other cardiovascular events. These authors17 also reported that Troponin I and NT-proBNP levels could provide prognostic information beyond currently used clinical score. Thus, at variance with previous observations where NT–proBNP levels were found to be elevated in AF patients in comparison to matched controls in sinus rhythm19 and sensitive to haemodynamic changes induced by the arrhythmia with a rapid normalisation after recovery of sinus rhythm,20 it was suggested that these parameters by reflecting the extent of atrial dysfunction could be utilised to identify patients at high thromboembolic risk.
Bleeding and Mortality in Atrial Fibrillation patients
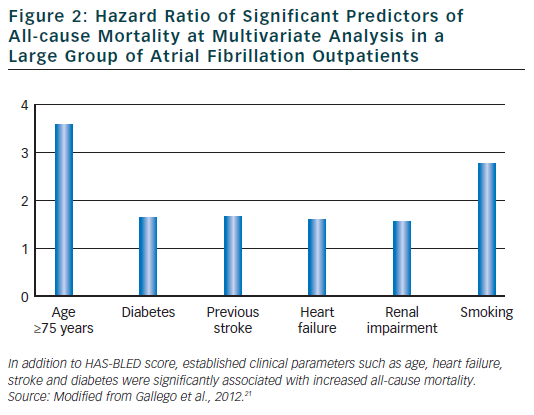
In spite of the significant reduction in thromboembolic risk induced by anticoagulant therapy, vitamin K antagonist are associated with bleeding.3 Many factors for thromboembolism such as, for example, advanced age, uncontrolled hypertension and cerebrovascular disease have also been identified as risk factor for bleeding. To facilitate clinicians in estimating major bleeding risk in AF patients, European guidelines3 have recommended the use of HAS-BLED risk score. Elderly patients who often have several co-morbidities and are taking several drugs are at increased bleeding risk. In these patients, haemorrhagic events are associated with major complications and mortality. It has been recently demonstrated21 that the HAS-BLED score measured in a large group of anti-coagulated AF out-patients was a significant predictor not only for major bleeding but also for adverse cardiovascular events including mortality. The score also predicted all-cause mortality.
In this large cohort of AF patients, independent predictors for adverse cardiovascular events were age >75 years, HF and stroke thus confirming the clinical utility and applicability of this simple clinical score in everyday clinical practice (see Figure 2).
When considering elderly patients at higher risk for both thrombotic and haemorrhagic events, it must be also recalled that anticoagulant therapy may also affect the number as well the severity of ischemic and haemorrhagic strokes,1–3 thus affecting not only the quality of life but also the mortality rate. As pointed out in a recent study of Fang and colleagues,22 carried out on a large group of patients with AF followed over a six-year period, warfarin was associated with a significant reduction of mortality and disability during an ischaemic stroke. Unfortunately, anticoagulant therapy increased mortality for haemorrhagic stroke. The advantages of anticoagulant therapy were particularly evident when appropriate therapeutic international ratios were maintained, whereas values >3 increased the odds of dying of intracranial haemorrhage.
Thus, in the decision process to start anticoagulant therapy for AF patients, not only the reduction in ischemic strokes but also the severity and associated mortality and disability have to be taken into consideration knowing that a minimal increase in intracranial haemorrhage cannot be avoided with warfarin. The possibility that this phenomenon might become less relevant with the new anticoagulant drugs is appealing but deserve further validation in the elderly populations.
Implantable Cardioverter Defibrillator Efficacy in Atrial Fibrillation Patients
Whereas implantable cardioverter defibrillator efficacy (ICD) therapy has been proven effective in reducing arrhythmic and cardiac mortality in several subsets of patients implanted for primary or secondary prevention, many recent studies have observed an increased mortality rate in patients receiving either appropriate or inappropriate ICD therapy in comparison to patients with no device interventions.23–25 This phenomenon is of particular relevance in ICD patients who presented AF either at the time or after ICD implantation. It has been recently shown24 that AF was the major contributor of inappropriate shocks in ICD patients with a rate greater than that due to lead defect or T-wave oversensing. Multiple ICD shocks triggered by AF were associated with a worse prognosis and were often observed in patients with other clinical co-morbidities such as worsening HF, electric storm or ventricular tachycardia, infection and exacerbation of severe obstructive pulmonary disease. Also in this subset of AF patients, the negative impact of inappropriate ICD shock due to AF was more likely to be related to the severity of the underlying cardiac conditions rather than on AF itself.26
Non-invasive Assessment of Mortality Risk in Atrial Fibrillation Patients
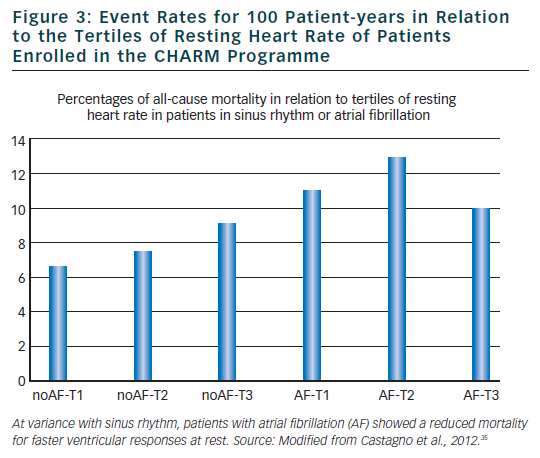
In sinus rhythm patients, identification of patients with an increased mortality risk has been successfully performed in different clinical conditions including acute myocardial infarction, heart failure, hypertension and diabetes. Among different approaches, those aimed to evaluate alterations autonomic modulations of sinus node27 and in ventricular repolarisation process28 have provided the most interesting results. For example, reduced heart rate variability, reflecting a diminished parasympathetic and an increased sympathetic modulation of sinus node has been associated with increased cardiac mortality after an acute myocardial infarction,27,29–32 whereas a negative micro T-wave alternans test has been found to identify post-myocardial infarction patients at very low arrhythmic risk.28 Most of the available methodologies that will not be discussed in the present article are based on the analysis of the duration or morphology of cardiac cycle during sinus rhythm being the regularity of RR interval a prerequisite for further analysis independently of the employed mathematical models. Unfortunately, irregularity of RR interval during AF prevents the possibility of using any of the available methodologies including time or frequency domain analysis of heart rate variability,27 baroreflex sensitivity,32 heart rate turbulence,33 micro T-wave alternans27 and T-wave variability.34 As a consequence, it is almost impossible to characterise the autonomic alterations and ventricular repolarisation abnormalities in AF patients. Regarding this point, it may be of interest to note that even heart rate loose its important predictive role in AF patients. A recent analysis of the CHARM study33 has confirmed that elevated resting heart rate was associated with worse outcomes in patients with HF and reduced LVEF. However, this relationship was not observed in patients with AF where, if anything, a higher heart rate was associated with a lower mortality (see Figure 3).
Conclusions
AF is associated with increased mortality is largely due to the degree of severity of co-morbidities that are present in AF patients. Ageing, HF and strokes are the most important prognostic factors and cause of death in these patients. Most of the available techniques used for risk stratification such as LVEF, NYHA functional class, resting heart rate provide less information in AF patients in comparison to those obtained in patients still in sinus rhythm.
As ageing is a non-modifiable parameter, reduction of disability and mortality in AF patients is based on prevention of HF progression and reduction of thromboembolic risk with an appropriate anticoagulant therapy.