The availability of technologies for real-time contact for sensing has significantly advanced and invigorated techniques of catheter ablation of AF, particularly with RF energy. This session was an in-depth look at contact force and included a critical perspective of the head-tohead with cryoballoon ablation. The experts discussed their opinions on the use of contact for sensing to reduce or avoid lesion gaps, and highlighted the significant and evolving body of evidence of clinical outcomes, as well as the caveats of too much contact force during the AF procedure.
Relevance of Fire and Ice
AF is the most common arrhythmia with a prevalence >33 million patients.20 Approximately 40% are asymptomatic,21 and another 30% are effectively treated with anti-arrhythmia drugs (AAD).22 Of the remaining 30% who are symptomatic and with failed AAD treatment,22 just 4% are treated annually23 – amounting to 396,000 treated with catheter ablation, and leaving 22.7 million patients who have not been effectively treated by drugs or undergone catheter ablation.22,23
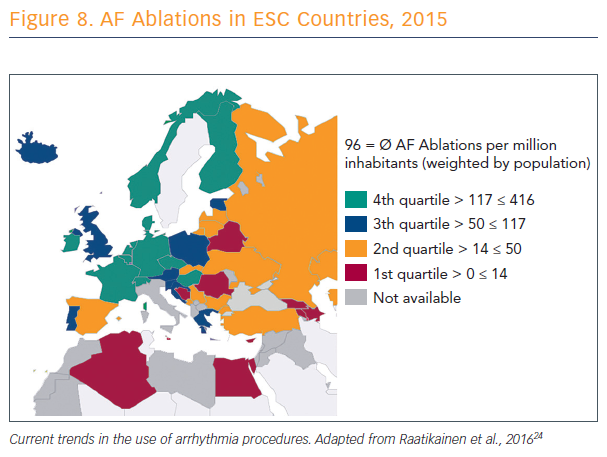
There is a lack of consistency across Europe in numbers and rates of catheter ablation procedures (see Figure 8).24 In these countries, said Prof Karl-Heinz Kuck of Asklepios Klinik St. Georg in Hamburg, Germany, electrophysiologists are discussing what to add to PVI. Countries with lower rates do not have access to catheter ablation or, if they have access, there are no clinicians available who know how to do it.
The development of balloon-based PVI is on the upswing, Prof Kuck suggested, because of the long learning curve, complexity, and challenge to create transmural, contiguous and permanent lesions with point-by-point ablation. He stressed that he is a strong supporter of CF-based catheter ablation, but this technique may not be optimal for every provider, especially given the widespread nature of AF.
There is no randomised controlled trial showing superiority of catheter ablation with contact force, compared to conventional RF catheter ablation, Prof Kuck explained. The only randomised trial that has compared a control group with a contact force group, aiming at non-inferiority, showed the same success rate when comparing the primary endpoints.25
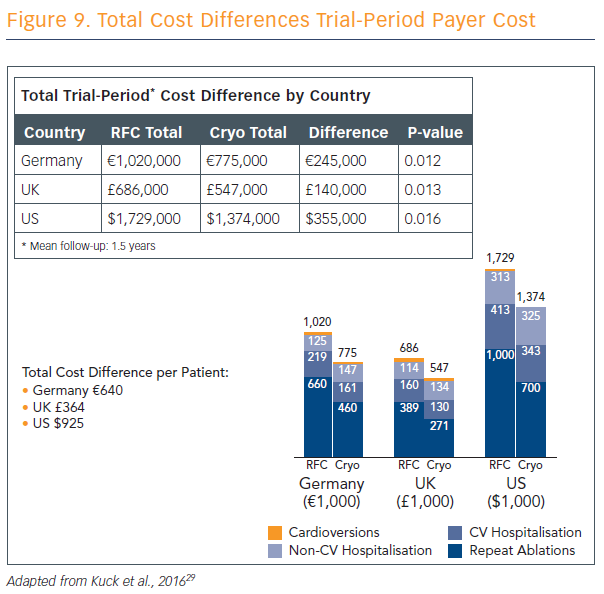
Ablation strategies that target the PVs and/or PV antrum are the cornerstone of most ablation procedures and, if the PVs are targeted, electrical isolation should be the goal. Furthermore, the new ESC guidelines recommend that catheter ablation should target isolation of the pulmonary veins using RF ablation or cryoballoon catheters.9 The radiofrequency catheter uses heat in a focal point-by-point delivery guided by electro-anatomical mapping, and cryoballoon uses freezing in a balloon single-step delivery guided by fluoroscopy without mapping. Prof Kuck explained the parameters for use of both techniques, and discussed the results of the largest cryoballoon versus RF ablation study ever performed and published.26 Cryoballoon ablation resulted in non-inferior mid-term efficacy compared with RF ablation at a median follow-up of 14 months, with significantly lower mean procedure times and lower left atrial (LA) dwell-time and lower costs (see Figure 9). Pericardial effusion was higher in RF patients compared with cryoballoon, but phrenic nerve injury was higher in cryoballoon and there were no incidences of phrenic nerve injury in the RF group. Mean fluoroscopy time was 2 minutes lower in the RF group.
“The cryoballoon-based PVI approach in paroxysmal AF is noninferior to the radiofrequency-based PVI with regard to frequency and safety, and superior with regard to re-hospitalization and re-ablation,” Prof Kuck concluded. “Novel balloon-based radiofrequency ablation systems combine direct visualisation of the anatomical substrate and, thereby, also new technologies for lesion formation. Permanent PVI can potentially be achieved to a high extent with a balloon technology… to my understanding, it’s the future of catheter ablation of AF, and the future is now.”
CF: What Does it Mean and How Does it Affect Lesion?
Traditional parameters of RF lesion control have been the target electrode temperature, delivered radiofrequency power in the power control mode, RF durattimes impedance-based control, and electrodetissue contact – an important but hitherto unquantified parameter. In the previous era, contact was evaluated in various semi-quantitative ways: fluoroscopy, tactile feedback, electrograms, electrode temperature and impedance, and intracardiac echo.
“It is only over the last 7 or 8 years that real-time contact force sensing has been available, which has given precise control of lesion creation,” said Prof Dipen Shah, from University Hospitals Geneva, Switzerland. Electrode tissue contact can be thought of as comprising of two components. The most important is the magnitude of the surface area of the electrode in direct contact with the tissue – the contact footprint.
Atrial tissue and the ventricle and myocardial tissue are soft, and as a rigid electrode is pushed into it, it gets more and more enveloped into the tissue. The second component is the stability of this contact, which can be considered as being composed of spatial stability (sliding of the tip electrode over the endocardium), and temporal stability, maintaining the intensity of the contact over time.
To quantify the contact pressure a catheter tip exerts on the tissue, the operator needs to know the exact surface area of contact. However, this cannot be assessed precisely. Experiments on ex vivo, porcine left atria showed that the same amount of force applied to different parts of the left atrium reduced the wall thickness by different amounts, depending on the thickness and tissue composition of the wall.27
A dynamic situation exists in vivo, including the effects of both cardiac and respiratory movement. To estimate a dynamically changing contact force over time, or at least its intensity, Prof Shah’s team formulated the area under the real-time contact force curve, termed the ‘force-time integral’, as a cumulative index of the amount of force over time. This allows, in one sense, a good measure of temporal stability, whereas spatial stability requires precise, two-dimensional localisation capabilities with respect not to a stable extra-cardiac spatial reference such as a back patch, but with reference to the intracardiac endocardia, which we do not yet have.
Research by Prof Shah and his colleagues determined the general effects of increased CF: increased electrode-tissue interface surface area; reduced electrode surface area exposed to (low impedance) blood; reduced electrode tip-sliding; tissue compression and thinning; tissue trauma; and higher tissue temperatures during RF delivery, including higher probability of a ‘pop’ and higher probability of extracardiac heating.28
To mitigate for risk, contact force sensors should be appropriately zeroed and re-zeroed after every reintroduction into the vascular system or through a sheath. It should be kept in mind that electromagnetic interference is likely to reduce the accuracy of this form of CF sensing. Catheter stability is important, and the composition of the atrial tissue should be kept in mind to reduce variances in lesion size.
Measuring CF remains important for recognising instances of absence of contact, reduction of ineffective ablations, better control of lesion size and repeatability, better arrhythmia-free outcome after PVI for paroxysmal atrial fibrillation (pAF) with optimal contact force, improved specificity for low-voltage substrate, reduced tissue trauma during catheter manipulation, and monitoring influence of respiration on contact, which Prof Shah thinks is an often-underestimated advantage of real-time CF sensing.
Determinants of Gap in CF-guided PV Encircling
Next, Prof Mattias Duytschaever from Bruges, Belgium, discussed CF–guided point-by-point PV encircling, and what determines a gap when CF has been used according to the ‘rules’. That is, if a patient has a reconnection in one or more veins, what has caused the gap, was there a weak link in the initial ablation chain and, finally, is it clinically relevant?
Prof Duytschaever presented an analysis of research on 42 paroxysmal AF patients who underwent contact force-guided PV encirclement. In the resulting 84 circles, 840 segments were identified, 44 of which had a gap. Segments with a gap were compared to durable segments without a gap.
Prof Duytschaever’s analysis defined the weakest link within each segment as the minimal time of application, power, delta impedance, CF, force-time integral and ablation index, and the maximal inter-lesion distance.
The ablation index is a formula that integrates power and contact force over time, taking into account the rapid rise of the lesion in the first seconds of the ablation, and a greater contribution of power.
“Inter-lesion distance seems straightforward, but don’t forget this is a new tool,” said Prof Duytschaever. “Before, we could not reliably measure inter-lesion distance because we were just putting tags during our ablation.
“The key finding: the difference between a segment with a gap and no gap is a lower ablation index. Vice versa, where all the ablation indexes were to a high level of specificity, the difference between segments with gap and no gap is the inter-lesion distance. They are independent; if you combine both parameters, if inter-lesion distance and the ablation index are more than 400 at the posterior wall and more than 550 on the anterior wall, you have a 93 % specificity for predicting a durable segment.”
Building on these data, Prof Duytschaever’s research group explored the use of enclosing the PVs with ablation index-guided RF applications with an inter-lesion distance of <6 mm and found the reproducible, perfect circle “invariably leading to PV isolation, and that makes a big difference. We [also] consider the presence of the oesophagus – an ablation index of 300 is enough if I have signs of oesophageal injury. That is most of the time an application of around 8 seconds.”
There were two complications in 250 patients using this approach, neither conclusively linked to the procedure, and no significant adverse events.
Prof Duytschaever’s group has submitted a paper on the comparative data of the last 50 CF-guided patients versus the first 50 close-guided ablations with the strict criteria. They have reached near-100 % acute isolation rate.
This is very reproducible; colleagues who are now using this technique, all acknowledge that this is reproducible and superior to CF-guided ablation, he said. Prof Duytschaever’s results show rates of freedom from AF at 3, 6, and 12 months of 92 % in the close-guided group.
Prof Duytschaever concluded that acute and late gaps in contact force–guided PV encircling are due to insufficient lesion depth and/ or discontiguity. The use of the strict-criteria ablation protocol results in a significant improvement in acute durability and arrhythmia-free survival. Nevertheless, real-time assessment of lesion formation remains one of the most important unmet needs in cardiac electrophysiology.
Randomised Trials of CF Efficacy: A Critical Look at the Literature
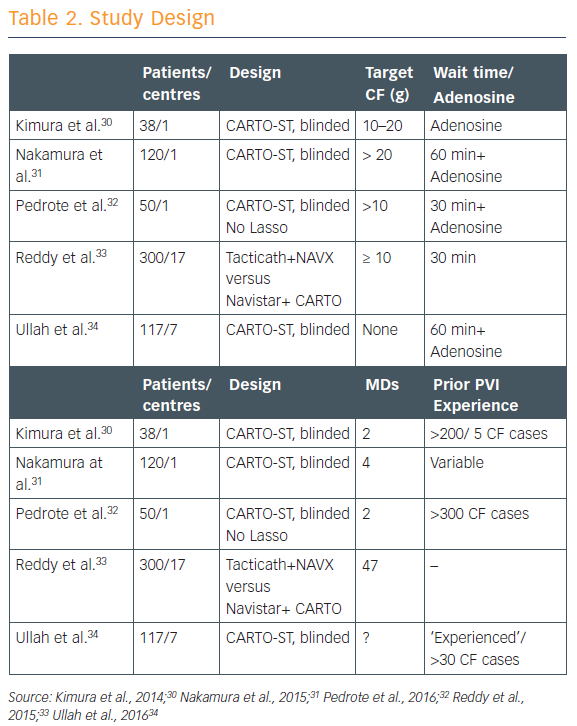
There have been five studies that have randomised patients to having the CF data either available to the operator or blinded to the operator (see Table 2).30–34 CF targets in each of the studies were ‘fairly modest’ at 5–20 g, and all except one involved a long waiting period of 60 minutes to watch for spontaneous reconnection. Most of these studies showed that using contact force shortened ablation time, decreased fluoroscopy time, and resulted in significantly fewer sites of acute reconnection. Complication rates did not differ significantly, but then these were infrequent overall.
“However, what really matters is how these patients did on follow-up, and [the existing randomised studies show] absolutely no improvement in clinical outcomes,” said Dr Dhiraj Gupta of Liverpool Heart and Chest Hospital in the UK.
“That’s really a big disappointment. Why have contact force trials not shown clinical success? Why couldn’t they prove what most operators believe strongly to be true?”
Analysis of the five trials raised two issues:
- long waiting time (30–60 minutes) – poorer quality lesions created without contact force got a chance to manifest themselves and be re-ablated, thereby negating much of the benefit; and
- mandating either no CF targets or low CF targets (<20g) – this meant that in most trials, the average CF between the two groups was similar.
“If we’re just concentrating on contact force alone we’re missing the point,” said Dr Gupta. “Contact force data help determine how deep each legion is, but it is equally important to have good contiguity between adjacent lesions. We know that force time integral is superior to contact force as a marker of lesion size. And power is an important component of legion creation. If you use high power, contact force becomes less and less relevant. And that’s where ablation index comes in. Contact force is critical in ablation delivery but, at the end of the day, it’s only one ingredient in what is a very complex recipe.”