Ventricular tachycardia (VT) is one of the most challenging medical conditions faced by cardiac patients and physicians treating them. Antiarrhythmic medications have limited effectiveness and are frequently poorly tolerated.1–4 Catheter ablation is increasingly used to treat patients successfully.1,5–7 Most VTs can be ablated endocardially but some require epicardial mapping and ablation. Electrophysiologists performing VT ablation need to know when to consider an epicardial approach, how to deal with anticoagulation during the procedure and ‘best’ techniques to minimise risk of procedural complications.
When to Suspect Epicardial Substrate
Patients with VT in the setting of ischaemic cardiomyopathy (ICMPY) generally have endocardial substrate that can be eliminated with endocardial radiofrequency lesions. Less commonly a critical component of the circuit is located epicardially in this setting and epicardial access is required. In contrast patients with non-ischaemic left or biventricular cardiomyopathy (NICM), who characteristically have perivalvular substrate, frequently have anatomic changes that either extend to the epicardium or are primarily epicardial in location, and require direct epicardial ablation.7–9 Other disease states with a high propensity for requiring epicardial ablation include patients with arrhythmogenic right ventricular cardiomyopathy (ARVC), cardiac sarcoidosis, Chagas disease and patients with a history of myocarditis.6,7,10–12 Patients with hypertrophic cardiomyopathy are unique in that apical scarring and epicardial substrate can be identified.13 Recently, there is evidence that epicardial right ventricular outflow tract may harbour substrate in patients with Type I Brugada syndrome and targeting this region may be beneficial to control VT/VF.14 The presence of any of these aforementioned disease states should raise the suspicion that epicardial m apping and ablation will be required.
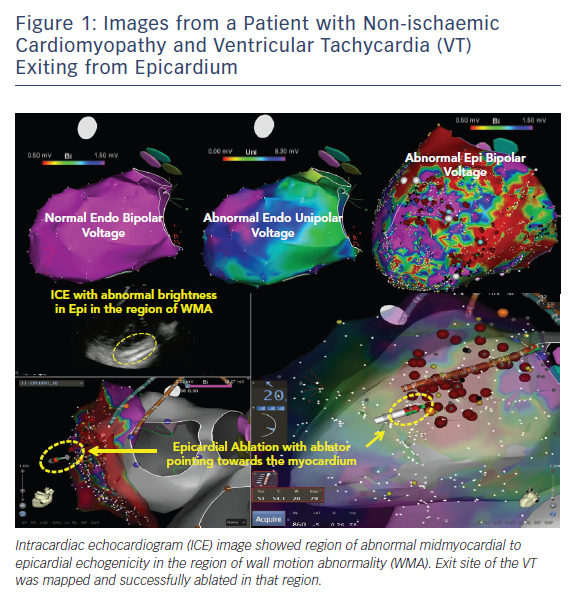
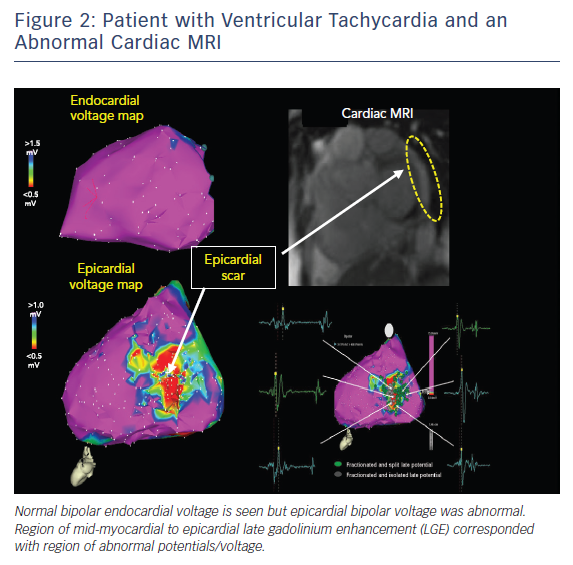
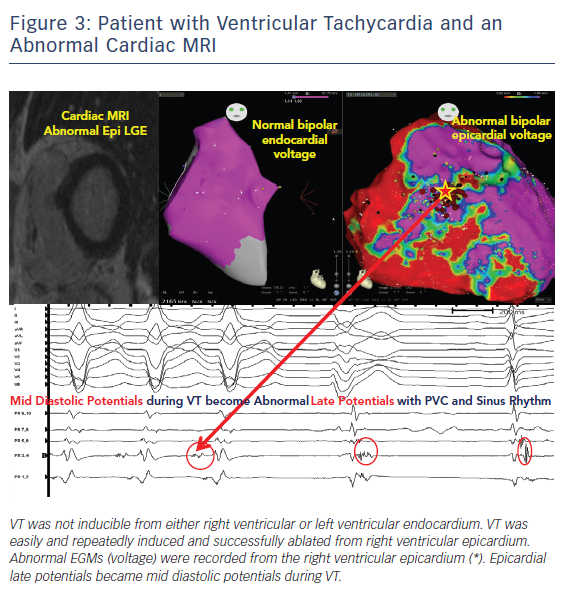
Pre-procedural planning is essential for any VT ablation. Cardiac imaging is performed most often prior to ablation procedures. Echocardiogram is useful in diagnosing the structural abnormality including regions of abnormal wall motion abnormality. Intracardiac echocardiogram (ICE) is useful during the procedure and can identify the region of abnormal substrate during the procedure (Figure 1). Cardiac MRI (CMR) is the best imaging modality currently available to localise the region of scar defined by late gadolinium enhancement (Figures 2 and 3). Electrophysiologists need to anticipate epicardial ablation when significant scar burden is localised to sub-epicardial or epicardial region.
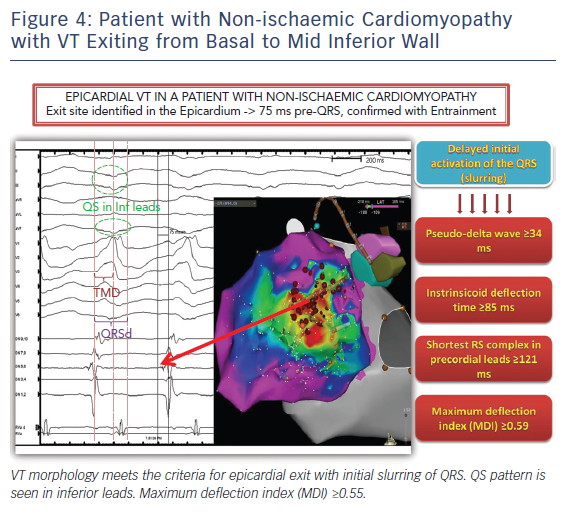
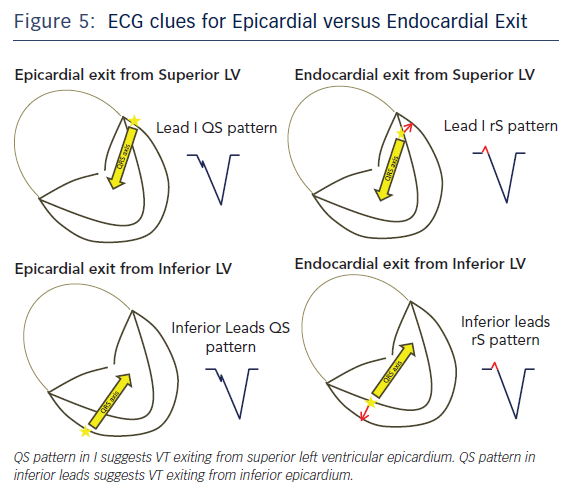
Twelve lead ECG recordings during VT hold vital clues to origin/exit of the VTs. The value of the 12-lead ECG for identifying epicardial origin is more limited in the setting of coronary disease in which the infarct produces activation delay with Q waves. The 12-lead ECG is most effectively applied in patients with idiopathic VT or VT in the setting of NICM to identify a probable epicardial origin. Wave fronts exiting the epicardium take longer to engage His-Purkinje fibres and therefore produce very slurred onset of QRS than wave fronts exiting from the endocardial VT circuits (Figure 4). When VTs exit from an endocardial region there is endocardial to epicardial activation which often forms a small R wave in the leads with the field of view facing the site of exit (Figure 5). An epicardial origin in patients with VT in the setting of NICM can produce a Q wave.15 Most of the criteria used to identify epicardial VTs is either interval or morphologic criteria. Slurring in the initial portion of QRS is identified with pseudo delta wave ≥34 ms, intrinsicoid deflection ≥85 ms and shortest RS complex in precordial leads ≥121 ms (Figure 4).16 Maximum deflection index (MDI) is calculated by dividing the shortest QRS onset to earliest maximum precordial deflection by total QRS duration. MDI values ≥0.55 also suggest epicardial idiopathic VT.15 A Q wave in lead I suggests that the VT is from superior left lateral LV epicardium while Q waves in II, aVF, III suggest that the VT may be from inferior epicardium (Figure 5).15 QS complexes in V2 can be seen with anterior epicardial exit originating from the LV summit region right under the region of the V2 lead.17
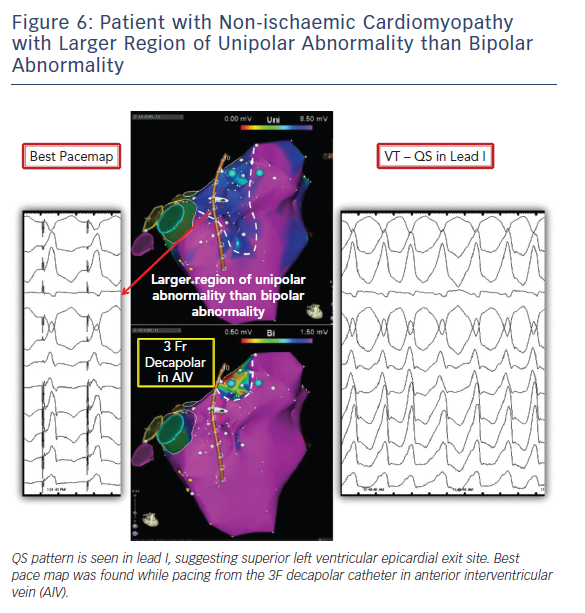
We routinely start the case mapping endocardially given the fact that many of the VTs originate from the endocardium or the circuit shares endo and epicardial components and the recognised challenges and possible complications faced with epicardial access and ablation. Endocardial voltage maps carry pertinent information about mid/epicardial substrate. Unipolar electrograms have a larger field of view compared to bipolar electrograms. This difference can be utilised to identify epicardial and midmyocardial substrate (Figures 1, 6 and 7). Normal unipolar voltage criteria were defined for left ventricle and right ventricular free wall, which are greater than 8.3 mV and 5.5 mV, respectively.18,19 Epicardial or midmyocardial scar is suspected when an area of unipolar abnormality is seen overlying normal bipolar voltage. A lower cut-off should be used when trying to identify deeper layers of scar through abnormal myocardium.11
Knowing when not to attempt epicardial ablation is as important as knowing when to attempt it in patients with non-ischaemic cardiomyopathy. When the endocardial voltage map suggests septal involvement and VT morphologies are consistent with either exiting from the septum or adjacent to the septum either superiorly and/or inferiorly, then an epicardial approach for ablating those intraseptal arrhythmias will not be successful. Trans-septal activation time measures the time taken to activate the opposite side of the septum from the site of pacing. Trans-septal activation time of more than 40 ms was specific for intramural conduction delay and the presence of intramural substrate.20 Occasionally complete compartmentalisation of right and left ventricular septum can occur and activation from right to left basal septum has to travel from RV base to normal apical region down the RV septum and then return up the left side of the septum from apex to base resulting in delayed activation over 90 ms in duration.20
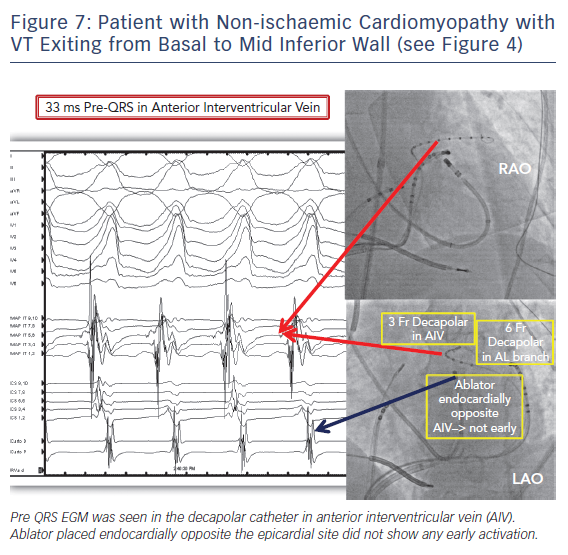
Left ventricular summit location is another challenging anatomic location with limited success rate with epicardial ablation. Ablation from the coronary venous anatomy may be limited by proximity to the coronary artery at the earliest site of activation in the distal great cardiac vein (GCV) or the anterior interventricular vein (AIV). Importantly, epicardial ablation targeting idiopathic VTs from the LV summit will frequently be limited by large proximal coronary arteries and a thick layer of fat and frequently ablation from adjacent endocardial aortic root or endocardium is preferred.21 Ablation from left ventricular endocardium or leftward aspect of right ventricular outflow tract will be ineffective if at a distance of more than 13 mm to the earliest site of activation in the coronary venous anatomy. VT from the more apical and lateral aspect of LV summit can be targeted epicardially. Successful sites met two out of three VT morphology criteria: 1) Q wave amplitude ratio in aVL/aVR >1.85; 2) R/S ratio >2 in V1; 3) no initial Q wave in V1.22
How to Deal with Anticoagulation During Epicardial Procedure
Guidelines recommend that pericardial access be obtained prior to systemic anticoagulation or after reversal of systemic anticoagulation.23 There are observational data for safely performing pericardial access in heparinised patients at high volume centres with experienced operators.24,25 However, the authors caution against such an approach until further studies with larger patient populations are available. Anticoagulation can be administered to allow further endocardial mapping and ablation if pericardial entry is achieved without bleeding or with modest bleeding that stops. Continued monitoring for pericardial bleeding is warranted after anticoagulation. Our target ACT for right-sided endocardial ablation is around 250–300 s and left-sided endocardial ablation is 300–350 s with a target of 350s needed if long sheaths are used in the arterial system.
How to Obtain Percutaneous Epicardial Access
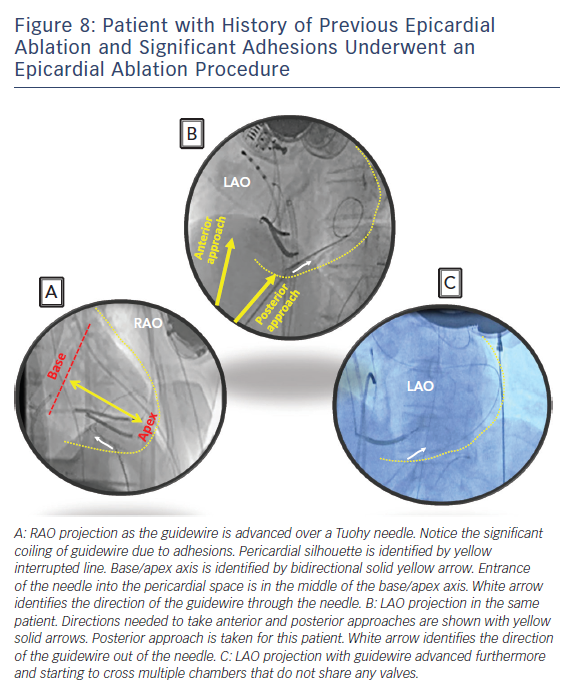
Subxyphoid percutaneous epicardial access for VT ablation was first popularised by Sousa and colleagues.26 Their technique is the most widely used to obtain percutaneous epicardial access with minor variations by individual electrophysiologists. We perform subxyphoid pericardial puncture with a 17G-Tuohy needle with few modifications to the original description. The patient is generally placed under general anaesthesia for comfort and safety but can be done under sedation as long as the patient is comfortable without major movements. The starting place for entrance into the skin is approximately 1 cm below the subxyphoid process for an anterior approach and right under the rib margin adjacent to the xyphoid for a posterior approach. Local anaesthesia is administered in this region. Next, a decision needs to be made whether to enter the pericardium with an anterior approach or a posterior approach. Entering the pericardium away from ablation target sites is favourable for catheter manipulation and stability. The anterior approach involves a shallow angle to the skin and the posterior approach involves a deeper angle. Steep left lateral fluoroscopic views for an anterior approach and a left anterior oblique (LAO) and right anterior oblique (RAO) fluoroscopic views for a posterior approach help target the region of entry safely. RAO view locates the needle in the bas to apical axis (Figure 8A). LAO view marks the direction of the needle in anterior to posterior axis (Figure 8B). It is generally safer to enter away from the base where major epicardial coronary arteries are found. Distal left anterior descending artery is found at the apex and therefore targeting just posterior to the middle of the base to apical axis may be the safest approach.
The Tuohy needle can be placed on the patient with the tip pointing towards the left shoulder and a brief fluoroscopic imaging in RAO projection will assess the angle of entry in the base to apical axis. Lifting the needle up and performing LAO fluoroscopy can assess the approximate angulation needed in anterior to posterior axis. Once the preliminary ‘best’ direction needed is assessed, the needle can then be advanced through the skin to the outer silhouette of the heart for few millimetres followed by removal of the stylet from the needle. At this point, a long guidewire is advanced through the needle. Small changes in the needle direction can be made as needed but we would recommend removing the needle all the way out of the body before major changes in the angle to prevent lacerating abdominal structures. The needle is advanced in small increments during expiration held 2/3 inspiratory effort when the patient is under general anaesthesia and the guidewire advanced out of the needle as soon as the needle penetrates the silhouette. When the needle reaches the heart border, ventricular pulsations will be transmitted from the needle tip. Premature ventricular contractions may also be seen. At this point a small contrast injection via the needle is optional and can identify tenting of the pericardium. Advancing the needle slightly with immediate advancing of the guidewire will safely obtain pericardial access. Often a ‘pop’ sensation is felt as the needle crosses the parietal pericardium similar to that noted when performing trans-septal puncture. Slight withdrawal of the needle once the guidewire is clearly within the pericardial space as one feels the ‘pop’ may reduce the risk of ventricular wall laceration. Most of the long guidewire should be advanced all around the pericardium. Fluoroscopy is used to identify the guidewire crossing multiple chambers that don’t share valves to rule out entrance in to the cardiac chambers. This is usually easiest to appreciate in the LAO projection as the wire will outline the silhouette of the heart border. Intracardiac echocardiography can be used to identify the guidewire in the pericardial space and to confirm that it is not in the cardiac chambers. At this point, a long sheath can be advanced over the guidewire. Deflectable sheaths can be useful during mapping and ablation in the pericardial space.
The mapping and ablation catheters are advanced to the pericardium though the sheath to perform mapping and ablation. Epicardial voltage maps need to be interpreted with caution. Dense epicardial fat decreases the voltage but the myocardium below it could be completely normal. These regions of dense fat are often found at the base of the heart and along major branches of the epicardial coronary arteries. Late potentials and abnormally fractionated electrograms are not normal and often represent a region of abnormal conduction and scar. The direction of the ablator is important when assessing voltage and or ablation. The mapping or ablation catheter needs to point towards the visceral pericardium and away from the parietal pericardium during mapping and ablation. Epicardial fat can also impede lesion delivery to the myocardium of interest. The ablation catheter energy delivery electrode can heat up quickly and reach a temperature cut off and limit power delivery due to lack of blood flow in the pericardium. Therefore, cooling the ablator tip with irrigation is recommended to deliver adequate power. The irrigation rate may not need to be as high as required in the endocardium and care must be maintained to adequately drain accumulating fluid to prevent cardiac compression.
How to Prevent and Manage Common Complications
Bleeding
Haemopericardium is a common adverse event seen with pericardial access, ranging from 5 % up to 30 % reported in literature.27,28 Pericardial bleeding can be categorised into early bleeding, bleeding during mapping and bleeding at the end of the procedure. Right ventricular puncture/laceration, coronary vessel puncture/laceration, and/or adhesion disruption are common reasons for early haemopericardium. Bleb rupture, multiple punctures especially in the setting of anticoagulation and steam pops with RF ablation can cause bleeding during mapping and ablation. Double right ventricular perforation could lead to extensive bleeding when the sheath is removed at the end of the case.
Prompt diagnosis, assessment of extent of bleeding and strategy for containing or fixing the cause is critical and can be life-saving. As such, ICE plays a crucial role for identifying and managing this complication in our laboratory (Figure 1). ICE can identify the location of guidewire when gaining initial access. Inadvertent puncture of the right ventricle is easily diagnosed when the guidewire is seen in RV with ICE. Most bleeding with RV punctures from the access needle stop bleeding without any intervention as long as the sheath is not advanced over the guidewire into the RV. Lacerations are more likely to continue to bleed and require surgical intervention. Similarly, most small vessel punctures or adhesion disruptions also stop bleeding without major intervention other than aspiration of the blood from the pericardial space. Major vessel puncture or chamber laceration requires cardiac surgery or interventional cardiology. For these reasons we recommend epicardial ablation to be done only when surgical backup is available. Each electrophysiology (EP) laboratory needs to have rapid anticoagulation reversal and blood transfusion protocols in anticipation of potential major bleeding complications. Despite taking all the necessary precautions, occasionally surgical rescue is warranted to fix a complication. Electrophysiologists are advised to take this opportunity to perform intraoperative surgical ablation once the acute bleeding problem is managed and stability is restored.
Prior coronary artery bypass surgery is a relative contraindication for epicardial ablation unless coronary anatomy is well defined and access to the VT circuit on the opposite side of the heart is possible. Prior cardiac valvular surgery is also a relative contraindication given the potential for significant adhesions that limit access and even if obtained, limits the ability to map freely.29 Repeated epicardial ablations and history of myopericarditis can lead to significant adhesions as well.29 Pericardial window by surgeons might be a safer approach when significant adhesions are anticipated.30
Hypotension during epicardial ablation requires close attention. The differential for hypotension includes tamponade, ECG changes or new wall motion abnormalities suggestive of coronary artery damage, drug reaction or anaesthesia-related complications. Intra-abdominal bleeding should be suspected when all of these are ruled out.
Phrenic Nerve
The course of the left phrenic nerve needs to be identified prior to performing epicardial ablation. High output pacing, 20–50 mA at 2 ms pulse width, is used to identify the region of phrenic nerve capture on the electroanatomical map.31 When the critical region for VT circuit is adjacent to the phrenic nerve, the phrenic nerve in the parietal pericardium can be displaced by inflating a pericardial balloon prior to ablation.32 Instillation of air or saline can also displace the phrenic nerve from the ablation target site.32 The left phrenic nerve can be captured by pacing from the left subclavian vein.33 Constant phrenic capture can be demonstrated and monitored while ablating in proximity to the nerve but persistent capture with pacing at low output warrants manipulation of the phrenic nerve away from the epicardial surface.
Epicardial Coronary Arteries
Ablating close to coronary arteries may lead to acute or chronic damage to these vessels.34 A coronary angiogram is routinely performed to outline the location of major epicardial vessels relative to the region of interest. The EHRA/ESC/ACC/HRS/AHA consensus statement on VT ablation recommends a distance of at least 5 mm away from coronary vessel for ablation before ablation is considered.24 Multiple angiographic views are recommended to assess the distance appropriately. Real-time integration of multidetector CT-derived coronary anatomy and CARTO-UNIVU has been utilised to better integrate the coronary anatomy with the electroanatomical map.
Pericarditis
Pericarditis is the most common adverse event of epicardial ablation. Adequate pain control immediately post procedure is required to minimise patient discomfort. Intrapericardial steroid injection at the end of the case has minimised pericarditis in in vivo animal studies35 and is commonly administered at a dose of 2–3 mg/kg of triamcinolone.
Conclusion
Epicardial ablation is frequently required to control VTs in patients who harbour epicardial substrate and VT circuit. It can be safely performed by experienced electrophysiologists with adequate surgical backup to manage uncommon bleeding emergencies. Preprocedure planning is mandatory to anticipate when to perform epicardial ablation and possible challenges and contraindications for the procedure. Awareness of procedure-related complications and techniques for their management including haemopericardium, intra-abdominal bleeding, damage to intra-abdominal organs, phrenic nerve injury, damage to coronary vessels and pericarditis helps to prevent these complications and manage patients safely during ablation when these complications do occur.
Clinical Perspective
- Preprocedural planning and preparation may identify the need for epicardial ablation for successful control of ventricular tachycardia with catheter ablation especially in patients with non-ischaemic cardiomyopathy.
- Twelve-lead electrocardiogram of ventricular tachycardia and ventricular bipolar and unipolar voltage maps contain valuable information to suggest epicardial exit and/or site of origin of ventricular tachycardia.
- Understanding, anticipating and preventing potential complications is vital for a successful and safe procedure when epicardial mapping and ablation is performed.