AF is the most common cardiac arrhythmia.1 AF has been estimated to occur in 20.9 million men and 12.6 million women worldwide, with a higher incidence and prevalence rates in developed countries.2 Estimates suggest an AF prevalence of approximately 3% in adults, with greater prevalence in older persons and in patients with underlying conditions, such as hypertension, heart failure, coronary artery disease, valvular heart disease, obesity, diabetes or chronic kidney disease.1,2 AF is an increasingly serious health problem in the ageing population. Regardless of the presence of comorbidities, AF is associated with a twofold increased risk of all-cause mortality in women and a 1.5-fold increased risk in men.2 AF has been identified as an independent risk factor for stroke, increasing the incidence fivefold.3
Stroke is the leading cause of long-term disability and is associated with high healthcare costs worldwide.4–6 It has been estimated that 20% of ischaemic strokes are caused by cardiac embolism, most often in the course of AF.7 According to the guidelines, it is recommended that the risk of thromboembolic complications is assessed on the basis of the CHA2DS2-VASc score.1,2,8 However, it is known that the CHA2DS2-VASc score identifies some, and not all, patients with an elevated risk of stroke or peripheral embolism.9,10 Despite a low CHA2DS2-VASc score, a patient may still experience stroke.11,12 In a meta-analysis from 2016, the summary estimate for the annual risk of ischaemic stroke was 1.61% (95% CI [0–3.23%]) for a CHA2DS2-VASc score of 1 and 0.68% (95% CI [0.12–1.23%]) for a CHA2DS2-VASc score of 0.13
The temporal relationship between stroke and AF is also an interesting issue. The current guidelines combine all types of AF with respect to anticoagulation, the main determinants of which are comorbidities that translate into risk markers.1,2,8,14 In the ASSERT trial, the temporal relationship of an episode of AF with the onset of stroke was unclear; very few patients had subclinical AF in the month before their event.15 This raises the question whether AF, rather than being a contributory factor, causes changes in atrial structure and endothelial function that are associated with the risk of stroke.15,16 Among patients in clinical trials, those with non-paroxysmal AF appear to have a greater risk of stroke than those with paroxysmal AF.14 Moreover, continuous monitoring of AF with cardiac implantable devices has provided us with the concept of the ‘AF burden’. Typically, the greater the AF burden, the greater the risk of stroke; however, this relationship is not well characterised in relation to the threshold above which the risk increases.14 In large cohorts of patients with cardiac implantable electronic devices and continuous rhythm monitoring, stroke risk increased the most in days 1–5 following an AF of ≥5.5 hours in duration, and diminished rapidly thereafter.17 In addition, AF episodes lasting >23 hours on a given day were associated with the highest risk of stroke.17 This indicates the complexity of the variables that may be associated with the risk of AF-related stroke.
Imaging studies are revealing the more patients with no history of transient ischaemic attack (TIA) or stroke have experienced cerebral infarction, and this is associated with an improved availability and quality of imaging techniques. Silent cerebral ischaemia (SCIs) is fivefold more frequent than stroke in the general population.18 Despite the absence of clinically significant symptoms of stroke, SCIs are associated with the occurrence and progression of neurological and cognitive deficits that commonly go unnoticed. Furthermore, the presence of silent infarcts more than doubled the risk of later stroke and dementia.18 MRI of the brain has shown a high incidence of cerebral ischaemia in asymptomatic patients with AF. Based on brain MRI studies, SCI is found in 92% of patients with persistent AF, and in 89% of patients with paroxysmal AF.19 However, in patients without AF, SCI occurs almost half as often, only in 46% of patients.19
Over 90% of embolic strokes are caused by thrombi originating from the left atrial appendage (LAA).20 For this reason, certain anatomical or functional parameters of the LAA could potentially be used to predict cardioembolic strokes and improve the performance of the CHADS2 and CHA2DS2-VASc scores.
Anatomy of the Left Atrial Appendage
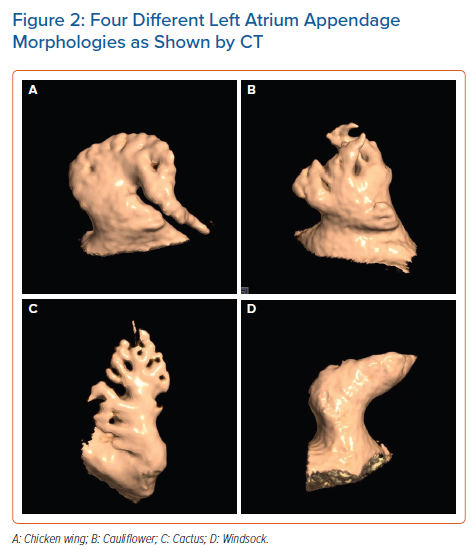
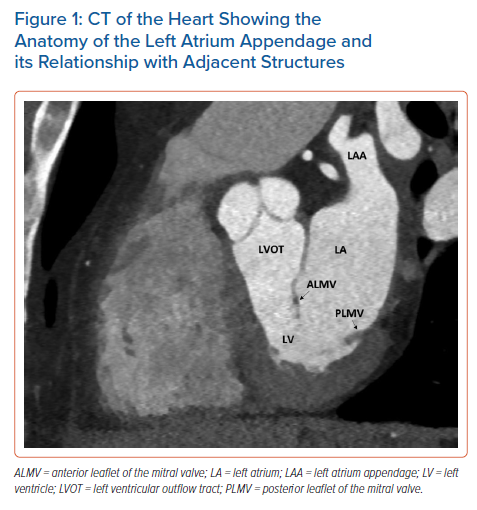
The LAA is a small, finger-like extension of the left atrium (LA). The LAA extends primarily between the anterior and lateral walls of the LA, parallel to the left pulmonary veins. The tip of the LAA is usually directed anterosuperiorly, overlapping the left border of the right ventricular outflow tract or the pulmonary trunk and the main stem of the left coronary or circumflex artery. However, it is not uncommon to find the tip of the LAA directed laterally and backwards (Figure 1).21,22 Despite a highly trabeculated endocardium, the LAA wall is very thin (~1 mm). The orifice of the LAA is usually oval. Round, triangular and water drop shapes are observed less frequently. A prominent ridge separates the orifice of the left pulmonary veins from the orifice of LAA, as well as the smooth muscular wall of the LA from the mitral annulus. The orifice leads to the LAA neck, which opens into the lobulated body. The LAA varies in size, number of lobes, shape, ostium and dimensions.22 In a large study of post-mortem hearts, Veinot et al. defined a lobe as a visible outpouching from the main tubular body of the LAA, usually demarcated by an external crease, with the tail itself also representing a lobe, although bends in the tail do not constitute new lobes.23 Veinot et al. found that two lobes were most common, with the number of lobes ranging from one to four, with the prevalence of one, two, three and four lobes being 20%, 54%, 23% and 3%, respectively.23 In 2010, Wang et al. classified the LAA morphology into four types based on CT and cardiac MRI – chicken wing, cauliflower, cactus and windsock (Figure 2).24
Function of the Left Atrial Appendage
The LAA has important neurohormonal and mechanical functions. The LAA has contractile properties, and its distensibility is larger than that of the LA. The endothelial cells of the LAA produce natriuretic peptides, namely atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP). Due to the greater extensibility and higher concentration of ANP in the LAA than in the LA, the LAA helps modulate LA pressure.25 Because natriuretic peptides play an important role in regulating fluid balance, filling the vascular bed and feeling thirsty, elimination of the LAA may impair this physiological regulation. Currently, there are limited data regarding the effect of LAA closure on plasma concentrations of natriuretic peptides.26–28 Experimental studies have shown that the mechanical activity of the LAA has no apparent effect on cardiac output, and therefore LAA occlusion or excision will not have any significant haemodynamic consequences.25
Undoubtedly, neurohormonal changes play a key role in the development of LA remodelling. Moreover, they also constitute the link between morphological changes and electrophysiological abnormalities that favour the triggering and maintenance of AF, creating a vicious circle between atrial cardiomyopathy and arrhythmias. Increased levels of a number of neurohormones, including ANP, BNP, angiotensin II and transforming growth factor-β1, are related to the relationship between atrial cardiomyopathy and arrhythmias.29 Mechanical stretching of the LA is the strongest factor stimulating increased ANP secretion.30 Two paracrine factors derived from endothelial cells play important roles in modulating ANP secretion, with endothelin enhancing ANP secretion and nitric oxide reducing it.30 ANP is a direct vasodilator that lowers blood pressure and inhibits the effect of renin, endothelin secretion, myocyte hypertrophy and collagen synthesis by fibroblasts.29 AF increases ANP concentrations, primarily as a result of haemodynamic effects, which indicates that the increase in ANP is an adaptive response to changing haemodynamic conditions in order to prevent remodelling.29,31
It has been reported that patients with a longer duration of AF have lower plasma ANP concentrations, suggesting that the reduction in ANP secretion in this population is most likely due to advanced atrial degenerative changes.32 BNP is an indicator of LA and left ventricular remodelling. Increased production of BNP occurs in response to increased stress on the heart wall as a result of volume and/or pressure overload. Such conditions are met in various phases of AF-related remodelling. In patients with AF, levels of BNP and N-terminal pro B-type natriuretic peptide are correlated with the type, and therefore duration, of arrhythmia.33 After electrical cardioversion of AF, a rapid decrease in BNP concentrations is observed, which may be the beginning, and also a marker, of reverse remodelling.29 It can be said that in the first phase of AF, biochemical remodelling takes place, which heralds structural remodelling.29
Angiotensin II plays a key role in the pathogenesis of AF-related remodelling by stimulating interstitial fibrosis. Angiotensin II may also mediate thrombus formation by interacting with thromboxane receptors and via nitric oxide- and prostacyclin-dependent mechanisms.29
It has been shown that in patients with AF, the endocardium of the LAA exhibits an elevated expression profile of prothrombotic and proinflammatory proteins compared with the right atrial appendage (RAA), indicating increased thrombogenicity of the LAA compared with RAA.34 This may explain, at least in part, the observation that most clots are formed in the LAA in patients with AF.34
Virchow’s triad is a group of well-documented factors explaining the pathophysiological mechanism predisposing individuals to the development of venous thrombosis.35 LA thrombosis likely has a similar background, with slightly different factors leading to its occurrence. Structural remodelling of the LA goes hand-in-hand with functional remodelling of the LA, which is primarily manifested by deterioration of contractility of the LA and LAA. A major indicator of functional LA remodelling is a reduction in blood flow velocity within the LAA. The lack of LAA contractility associated with AF leads to blood stagnation, which promotes the formation of a thrombus. There are several mechanisms during AF that promote thrombogenesis: endocardial damage by atrial dilatation, endocardial denudation, fibroelastic infiltration of the extracellular matrix, haemostatic and platelet activation and growth factor changes during AF. All these factors complete the triad.25
Morphological and Haemodynamic Features of the Left Atrial Appendage and the Risk of a Thromboembolic Event
The LAA is a major thromboembolic source in patients with AF. Therefore, many studies have assessed the risk of stroke by analysing morphological and haemodynamic features of the LAA (Supplementary Material Table 1). There are three known morphological features of the LAA associated with ischaemic stroke: shape, size and fibrosis.36
A specific morphology of the LAA has been reported to be related to stroke in patients with AF. In 2012, Di Biase et al. studied the LAA by CT and MRI to classify different LAA morphologies and correlate the morphology with the history of embolic cerebrovascular events in patients with AF.37 The prevalence rate of stroke or TIA among the study population was 12% for those with a cactus morphology, 4% for those with a chicken wing morphology, 10% for those with a windsock morphology and 18% for those with a cauliflower morphology (p=0.003). The study showed that patients with a chicken wing morphology were less likely to have an embolic event after adjustment for comorbidities and CHADS2 score, whereas the cauliflower morphology appeared to be the most frequently associated with thromboembolism.37
Anselmino et al. investigated the correlation between LAA morphology studied by MRI or CT and the SCI detected by cerebral MRI in patients with AF undergoing transcatheter ablation (SCI was detected in 84.8% of patients).38 That study showed that LAA morphology was associated with SCI in AF patients. Patients with a chicken wing LAA morphology had a significantly lower risk of SCI, whereas those with windsock or cauliflower LAA morphology were at an increased risk of SCI.38 Lupercio et al. conducted the first meta-analysis, including eight studies and 2,596 patients (84% with a CHADS2 score <2), to determine thromboembolic event risk for each LAA morphology in patients with AF with low to intermediate risk.39 The authors showed that chicken wing LAA morphology was associated with a lower risk of thromboembolic events than non-chicken wing morphology.39 This was confirmed by other authors.40 In another study, Wang et al. showed that the number of LAA lobes is an independent risk factor and has a moderate predictive value for LA thrombus and LA spontaneous echo contrast among patients with AF factors.41
In addition to LAA shape, LAA size is also thought to play a role as a predictor of stroke. LAA dimensions assessed using multiple imaging modalities have been directly correlated with thromboembolic risk. For example, Beinart et al. showed that higher LAA volume (mean [± SD] 22.9 ± 9.6 cm3 versus 14.5 ± 7.1 cm3; p<0.001), larger LAA depth (3.76 ± 0.9 cm versus 3.21 ± 0.8 cm; p=0.006) and long (3.12 ± 0.7 cm versus 2.08 ± 0.7 cm; p<0.001) and short axes (2.06 ± 0.5 cm versus 1.37 ± 0.4 cm; p<0.001) of the LAA neck were associated with an increased risk of a history of stroke, and that LAA neck measurements (short axis × long axis) had the strongest correlation with stroke risk.42 Burrell et al. focused on the LAA volume, measured with MRI, in AF patients with or without a history of stroke.43 In that study, patients with a history of stroke had larger LAA mean volumes than control subjects (28.8 ± 13.5 cm3 versus 21.7 ± 8.27 cm3; p=0.002), and stroke risk was highest in patients with an LAA volume >34 cm3.43 In contrast, Di Biase et al. did not find the size of LAA to be a predictor of stroke.37
A larger LAA orifice area has also been shown to be associated with ischaemic stroke.44–46 Lee et al. found that patients with stroke had a larger LAA orifice area (mean ± SD 4.5 ± 1.5 cm2 versus 3.0 ± 1.1 cm2; p<0.001) and showed that LAA size was the most powerful predictor of stroke in patients with low CHA2DS2-VASc scores.44,45
Khurram et al. studied the association of LAA morphology and the extent of trabeculations, as well as orifice diameter and length, with prevalent stroke.47 The extent of LAA trabeculations (27.7% versus 14.4%; p=0.019) and a smaller LAA orifice diameter (mean [± SD] 2.26 ± 0.52 cm versus 2.78 ± 0.71 cm; p<0.001) were associated with a higher risk of stroke, and this may explain the association between cauliflower LAA morphology and stroke.47
The orientation of the LAA to adjacent cardiac structures has also been shown to be an additional risk factor for the presence of thrombus. Nedios et al. found an association between a higher LAA take-off (i.e. higher than the left superior pulmonary vein) with a tachycardia-mediated thrombogenic flow and an increased thromboembolic risk in patients with AF and a low CHA2DS2-VASc score.48 In another study, Dudzińska-Szczerba et al. showed that the distance from the LAA ostium to the first bend of the LAA was independently associated with stroke risk in patients with AF.49
AF is also known to be associated with structural remodelling of atrial tissue and fibrosis. This remodelling process is an integral part of the pathophysiology of arrhythmia and is necessary for a chronic arrhythmia to occur. Atrial fibrosis in patients with AF may play a significant role in the development of electromechanical dysfunction leading to stasis and localised contractile dysfunction that may form the focus of thrombus formation.50 Akoum et al. used late gadolinium enhancement cardiac MRI to detect structural LAA dysfunction in patients with AF, finding that LAA fibrosis was associated with reduced LAA flow velocities, and thus with blood stasis, thrombus formation and the risk of stroke.51,52
In addition to LAA morphology, LAA function has been associated with an increased risk of stroke in patients with AF. Lower LAA velocities have been shown to correlate with ischaemic stroke and thrombus formation. Post hoc analysis from the SPAF-III trial, which included 721 patients who underwent transoesophageal echocardiography (TOE), showed that peak LAA anterograde flow velocity <20 cm/s was independently associated with thrombus formation and the risk of cardioembolic stroke.53 Uretsky et al. found that mean LAA contraction velocity was lower in patients with LAA thrombus than in those without (10 ± 4 cm/s versus 22 ± 7 cm/s; p<0.001) and lower in patients with AF and a history of stroke/TIA than in those without (11 ± 3 cm/s versus 15 ± 6 cm/s; p=0.008).54 Moreover, Uretsky et al. noticed that nearly one-third of patients with LAA flow velocity (LAAFV) ≤11 cm/s had LAA thrombus.54 Several studies have reported complex interactions between LAA haemodynamics and geometry. For example, Lee et al. evaluated whether a specific LAA morphology was related to stroke and whether it was related to haemodynamic changes and the size of the LAA in AF patients.45 That study confirmed that a chicken wing LAA was associated with a decreased stroke risk. Moreover, it was shown that patients with a chicken wing LAA have a smaller LAA orifice area and higher LAA velocity than those with a non-chicken wing LAA. Thus, Lee et al. suggested that an increased LAA orifice and decreased LAAFV may be associated with increased stroke risk.45 In another study, Lee et al. observed that the presence of both increased LAA orifice area and decreased LAAFV were significant risk factors for stroke.44 Among patients with an LAAFV <37.0 cm/s, patients with a large LAA orifice (>3.5 cm2) had a greater incidence of stroke than those with an LAA orifice ≤3.5 cm2 (75% versus 23%; p<0.001).44
Others have come to similar conclusions. For example, one study showed that cauliflower LAA morphology was the most common morphology in patients with cardioembolic stroke (CES) and the least common in patients with AF and no history of cerebrovascular accident (AF/non-CVA); in contrast, chicken wing LAA morphology was most common in AF/non-CVA and the least prevalent in CES patients.46 Furthermore, LAA orifice diameters were larger in the cardioembolic TIA (CETIA) and CES groups than in the AF/non-CVA group. LAAFV was higher in the CES group than in the other groups. Multiple multinomial regression analyses showed that the cauliflower morphology was associated with CETIA and CES; however, after adjusting for LAA orifice diameters and LAAFV, LAA morphology was no longer associated with either CETIA or CES.46 Receiver operating characteristic curve analysis showed that LAA orifice diameter and LAAFV accurately predicted CETIA and CES.46 In another study, significant differences were found in LAAFV between the specific LAA morphologies.55 LAAFV was higher in patients with chicken wing LAA than in those with cactus and cauliflower LAA. After adjusting for covariates, the study revealed that LAA morphology was a significant determinant of LAAFV and suggested that the relationship between a specific LAA morphology and stroke may also be explained, in part, by the change in LAAFV.55
There is continuous research for new markers of LAA dysfunction. In a recent study, Jankajova et al. analysed the significance of LAA deformation in relation to thromboembolic events using TOE.56 In that study, the velocity vector imaging-derived LAA strain rate was found to be a significant predictor of ischaemic stroke and systemic thromboembolism in patients with AF, with predictive power similar to the CHA2DS2-VASc score.56
Left Atrial Appendage Occlusion
Oral anticoagulation (OAC) is the standard therapy for stroke prevention in AF, but its use is associated with a risk of bleeding and its effect depends upon patients’ adherence to recommended treatment.
LAA occlusion (LAAO) provides an alternative to OAC for thromboembolic risk reduction in patients with non-valvular AF (NVAF). The most common rationale for LAA closure/exclusion in clinical practice is the observed high risk of bleeding or, less frequently, contraindications to the use of OAC, as well as the occurrence of ischaemic stroke during anticoagulant therapy.1
Evidence from three randomised control trials supports LAAO.57–59 Specifically, the WATCHMAN device (Boston Scientific) was compared to vitamin K antagonist therapy in randomised control trials (PROTECT AF and PREVAIL). These studies found that LAA closure was not inferior to stroke prevention treatment with vitamin K antagonist therapy in patients with AF with a moderate risk of stroke, with a possible reduction in bleeding during longer follow-up.57–59
A meta-analysis including patients from the PROTECT AF and PREVAIL trials and their respective registries reported that, in patients with NVAF who were at increased risk of stroke or bleeding, LAAO, compared with warfarin, resulted in improved rates of haemorrhagic stroke (0.15 versus 0.96 events/100 patient years; HR 0.22; p=0.004), cardiovascular/unexplained death (1.1 versus 2.3 events/100 patient years; HR 0.48; p=0.006) and non-procedural bleeding (6.0% versus 11.3%; HR 0.51; p=0.006).60 Although there was a greater incidence of ischaemic stroke in the device group (1.6 versus 0.9 events/100 patient years; HR 1.95; p=0.05), the rates of ischaemic stroke were no longer significantly different between the device and warfarin groups after exclusion of procedure-related strokes.60
As direct oral anticoagulants (DOACs) also provide a significant reduction in haemorrhagic stroke and mortality compared with warfarin, the question may be raised as to whether LAAO or DOAC therapy may be more appropriate for a given patient. This issue was recently raised in the non-inferiority study PRAGUE 17. Among high-risk patients with NVAF, LAAO use was not less effective in preventing cardiovascular or neurological events than DOAC.61
A recent meta-analysis compared the results of the three currently available randomised control trials assessing LAAO versus OAC, namely the PROTECT AF, PREVAIL and PRAGUE 17 trials.62 That analysis found no significant differences in ischaemic stroke (RR 1.48; p=0.13) or overall major bleeding (RR 0.89; p=0.46) between groups. Compared with OAC, LAAO provided a significant reduction in haemorrhagic stroke (RR 0.22; p=0.002), non-procedural major bleeding (RR 0.53; p<0.001), cardiovascular death (RR 0.65; p=0.03) and all-cause death (RR 0.78; p=0.04).62
In addition, multiple registries have reported favourable outcomes for the WATCHMAN, Amplatzer Cardiac Plug and Amulet devices (Abbott) in patients with a higher bleeding risk and contraindications to short-term OAC for whom less intensive post-LAAO anticoagulation regimens were required.63 There is also growing evidence of the efficacy and safety of newer devices with distinct characteristics that may be of value to specific patient subgroups.63
Numerous observational studies have demonstrated the feasibility and safety of LAA surgical closure/shutdown. Residual LAA flow or incomplete LAA closure may be associated with an increased risk of stroke. In a multicentre randomised trial involving participants with AF and a CHA2DS2-VASc score of at least 2.0, who had undergone cardiac surgery and most of whom continued to receive ongoing antithrombotic therapy, stroke or systemic embolism occurred in 4.8% of participants in the occlusion group, compared with 7.0% in the non-occlusion group (HR 0.67; 95% CI [0.53–0.85]; p=0.001). The incidence of perioperative bleeding, heart failure or death did not differ significantly between the trial groups.64
There is a need for studies of adequate power to determine the best indications for LAAO or LAA exclusion versus DOAC therapy in patients with relative or absolute contraindications to anticoagulant therapy and in patients with ischaemic stroke during anticoagulant treatment, as well as to evaluate appropriate anticoagulant treatment after LAA closure.
Right Atrial Appendage and the Risk of Embolism
Although thrombi migrating from the LAA are usually blamed for peripheral embolisation of cardiac origin, the RAA may occasionally be the source of embolism. Currently, the literature lacks detailed data on the anatomy, function and incidence of thrombus in the RAA. Pulmonary embolism (PE) and paradoxical migration through the patent foramen ovale with a risk of systemic embolism, including stroke, are potential complications of thrombus in the RAA.65 However, the clinical relevance and the incidence of thrombus in the RAA are not well understood.66
The RAA is a triangular extension of the right atrium (RA) with a mean area of 3 cm2 that wraps around the aortic root and is composed of a trabecular network of pectinate muscles.67,68 The sagittal bundle connects the terminal crest with the apex of the RAA.68 The following appendage shapes have been identified: horse head, parrot beak, anvil, sailboat and indeterminate. The number of lobes ranges from one to six.69
The risk of thromboembolic events has been shown to increase with morphological complexity, larger surface area and dysfunction of the RAA. The presence of spontaneous echo contrast in the RA is also an independent predictor of thrombus formation in the RAA.70
Based on data from TOE, thrombi are much less common in the RAA than in the LAA, with an incidence, according to various reports, that ranges from 0.5% to 0.8% in AF/atrial flutter patients (versus 5.9–10.3% in the LAA).71,72 It seems that the lower incidence of thrombus formation in the RAA compared to LAA may be due to differences in the morphology and anatomical location of the two structures. The wide RAA ostium may facilitate thrombus migration. Conversely, the location of the RAA adjacent to the inlet of the superior vena cava may reduce the coagulation capacity. The lower reported number of RAA thrombi in TOE may also be secondary to problems with the visualisation of this structure.73
One study, which included a total of 23,796 autopsies, suggested that the incidence of thrombus in the RA was the same as in the LA, reaching 3.1%. However, there were no data as to the proportion of thrombi located in the appendages.74
Another controversial and often overlooked issue in clinical practice is PE in patients with AF.66 In the previously mentioned study, it was shown that 7% of patients who died of PE had a right-sided intracardiac thrombus and 62% had no other potential source of embolisation.74 The incidence of AF in PE patients ranges from 15% to 21% and is much higher than in the general population.73 It has been shown that the occurrence of AF is associated with a significantly higher risk of another episode of venous thromboembolism compared with patients with sinus rhythm.73,75 Several studies have shown that patients with PE without concomitant deep vein thrombosis more often have a history of AF than patients with PE associated with deep vein thrombosis.76,77 The relationship between AF and PE has a pathophysiological background. The stagnation of blood in the atria, along with the pectin muscles, is a substrate for thrombus formation in AF. However, there are insufficient clinical data on the relationship between these two conditions. This could be for a variety of reasons, including the inability to visualise the RAA using TOE, which is routinely performed after a diagnosis of PE, and the lack of recommendations for TOE examination in this patient group. Therefore, it can assumed that the actual incidence of thrombus in the RAA in PE patients has not been established. Another reason may be that episodes of PE often remain clinically silent in patients with AF. In addition, most studies of right heart thrombosis did not analyse the incidence of AF in this population.73
Paradoxical embolism in the presence of patent foramen ovale may be another manifestation of the RAA emboli. Data in the literature are scarce, but some authors have reported that RAA function is altered in relatively young patients with patent foramen ovale and cryptogenic stroke.78 Thus, searching for RAA thrombi in patients with patent foramen ovale and cryptogenic stroke or peripheral arterial embolism may be advocated in selected patients.
In summary, the treatment of patients with RAA thrombi is currently unclear. Some authors suggest that the management of thrombus in the RAA should be the same as for LAA thrombus. It has been proposed that in all patients undergoing TOE for LAA thrombus evaluation prior to cardioversion, thrombus in the RAA should also be excluded.66,71,72 If PE occurs in a patient with AF and without deep vein thrombosis, screening for RAA thrombus by TOE should also be performed.66 However, the optimal management of right-sided thrombi remains unclear.
Conclusion
Numerous studies have shown that some anatomical and functional LAA features are independently associated with stroke prevalence in a population of patients with AF and could improve the performance of the CHAD2 and CHA2DS2-VASc scores. However, the results of studies are often contradictory (e.g. LAA shape and stroke risk). None of the LAA parameters has been universally accepted as a strong risk factor or included in the currently used stroke risk scales. Thus, more research is needed to establish the role of LAA parameters in stroke risk assessment in patients with AF. Percutaneous LAAO can be a safe and effective non-pharmacological treatment option for patients with NVAF.
Click here to view Supplementary Material.
Clinical Perspective
- The European Society of Cardiology guidelines recommend a thromboembolic event risk assessment based on the CHA2DS2-VASc score in patients with non-valvular AF; however, stroke occurs also in some patients with low CHA2DS2-VASc scores.
- The leading cause of embolic strokes is thrombi originating from the left atrial appendage (LAA); therefore, some anatomical or functional parameters of the LAA could potentially be used to predict cardioembolic stroke and improve the performance of CHA2DS2-VASc scores.
- New factors need to be found to improve thromboembolism risk stratification in the AF patient population.
- LAA occlusion provides an alternative to oral anticoagulation for thromboembolic risk reduction in patients with non-valvular AF.