Over the past decades, the life expectancy of children born with congenital heart disease (CHD) has improved considerably.1,2 Hence, the population of adult CHD (ACHD) patients is growing.3 Many ACHD patients are affected lifelong by cardiac symptoms, reduced quality of life and cardiac events.2 Clinical arrhythmias are important regarding morbidity and mortality in patients with ACHD.4–6 Clinical arrhythmias and subclinical arrhythmias are crucial signs in the management of ACHD patients due to their potential impact on their overall cardiovascular health. Clinical arrhythmias, with noticeable symptoms, can significantly affect quality of life, and may necessitate prompt intervention to prevent complications. Subclinical arrhythmias, although asymptomatic, may be an early sign of deterioration, and can still contribute to increased morbidity and mortality. Therefore, regular monitoring and timely intervention is essential in the comprehensive care of ACHD patients.1
It is estimated that one out of six ACHD patients develops bradycardia or tachyarrhythmia during life, that often precede syncope and/or sudden death.5 For example, more than one-third of tetralogy of Fallot (TOF) patients develop symptomatic atrial tachyarrhythmia as adults, and 10% develop high-grade ventricular arrhythmia. Moreover, of these TOF patients, 5% require a pacemaker implantation for surgically acquired atrioventricular block or sinus node dysfunction.3 To prevent sudden cardiac death, adequate risk assessment is needed to identify patients at high risk for sudden cardiac death. In TOF patients, mortality and ventricular arrhythmia are associated, among other clinical parameters, such as older age at repair, prior palliative shunt, longer QRS duration, at least moderate right ventricular function, lower left ventricular ejection fraction, previous ventriculotomy and higher right ventricular end-diastolic volume. The more these factors are present in a patient, the more repeated monitoring for detecting arrhythmias becomes important.7
In patients with Senning or Mustard repair for transposition of the great arteries – a group declining in size – loss of sinus rhythm is demonstrated in 60% of patients. The risk of atrial and ventricular arrhythmias increases with age.8 At every arrhythmic event, symptomatic or asymptomatic, a change in patient management should be considered; for example, initiation of anticoagulation, change in antiarrhythmic drugs, catheter ablation, or implantation of a pacemaker or ICD. Also, evaluation of possible underlying structural abnormalities, which may be causal and correctable, is an essential part of clinical management for patients with arrhythmias.
ACHD patients are mainly cared for in outpatient clinics, where brief evaluations of clinical status, patient education and treatment strategies during consultation are made.9 These outpatient evaluations are only momentary snapshots, ranging from a few times a year to once every 5 years.
The most recent European Society of Cardiology guidelines for the management of ACHD patients advise screening for arrhythmia if patients are symptomatic or for selected patients if bradycardia and/or tachyarrhythmias are suspected.2 In ACHD patients with pacemakers or implantable cardioverter defibrillators, arrhythmias can be detected by device interrogation.10,11 Holter monitors are used to perform screening for arrhythmias or assess arrhythmia burden for a short term. Instruments to perform extended ambulatory heart rhythm monitoring are increasingly available. The aim of this review was to assess the added value of these instruments in respect to detecting arrhythmias in patients with ACHD, leading to meaningful changes in clinical care.
Methods and Definitions
A PubMed database search was performed. Relevant search terms regarding CHD were combined with search terms regarding screening instruments and adults. The search domain was restricted to January 2007 to October 2022. This restriction on publication dates prior to 2007 was used because of the fast development of electrophysiology techniques over the past decades. Additionally, the search was restricted to publications in English language. Four categories were created: cumulative Holter findings, 2-week continuous monitor, smartwatch- and smartphone-based single-lead ECG; and implantable loop recorder (ILR).
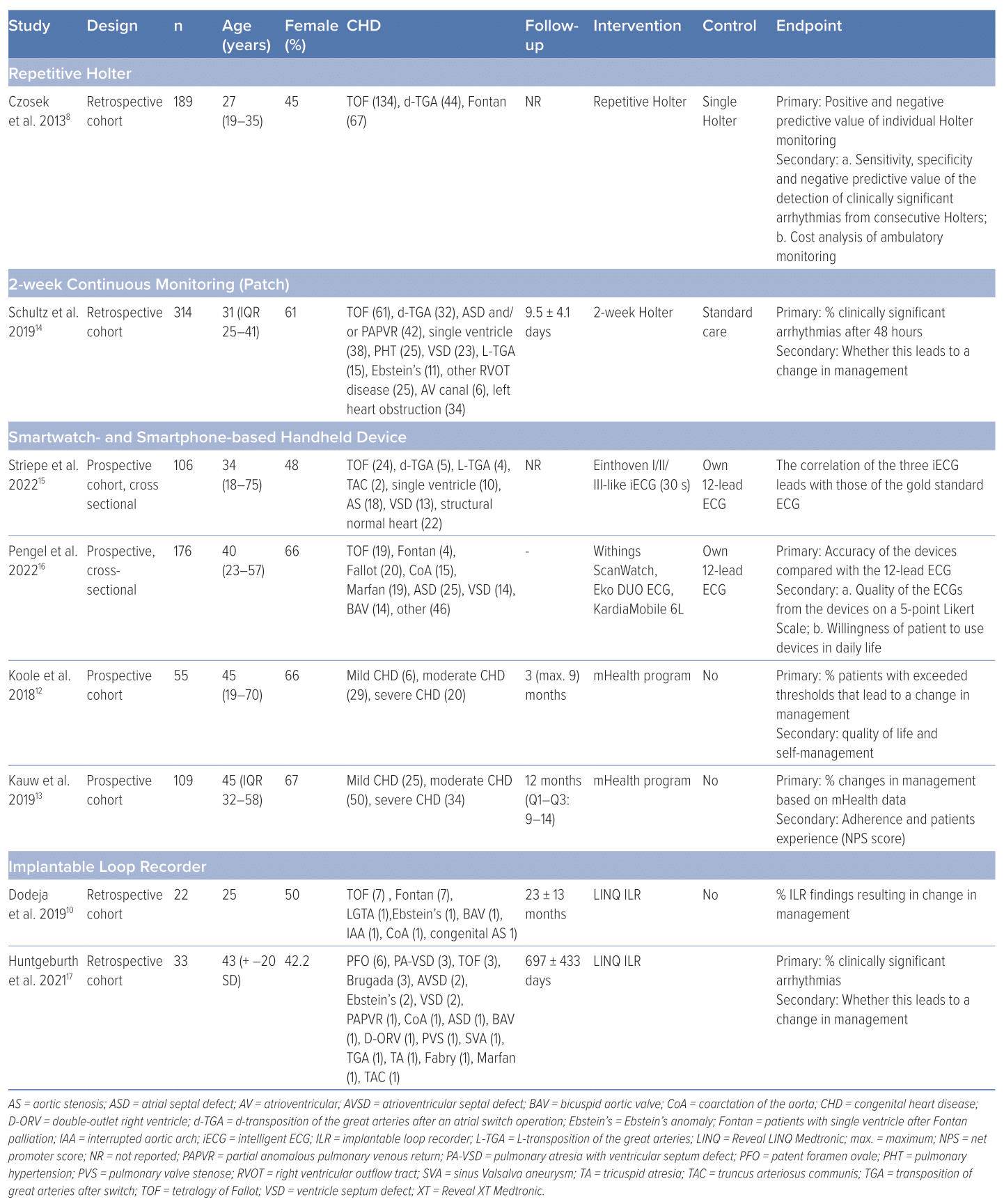
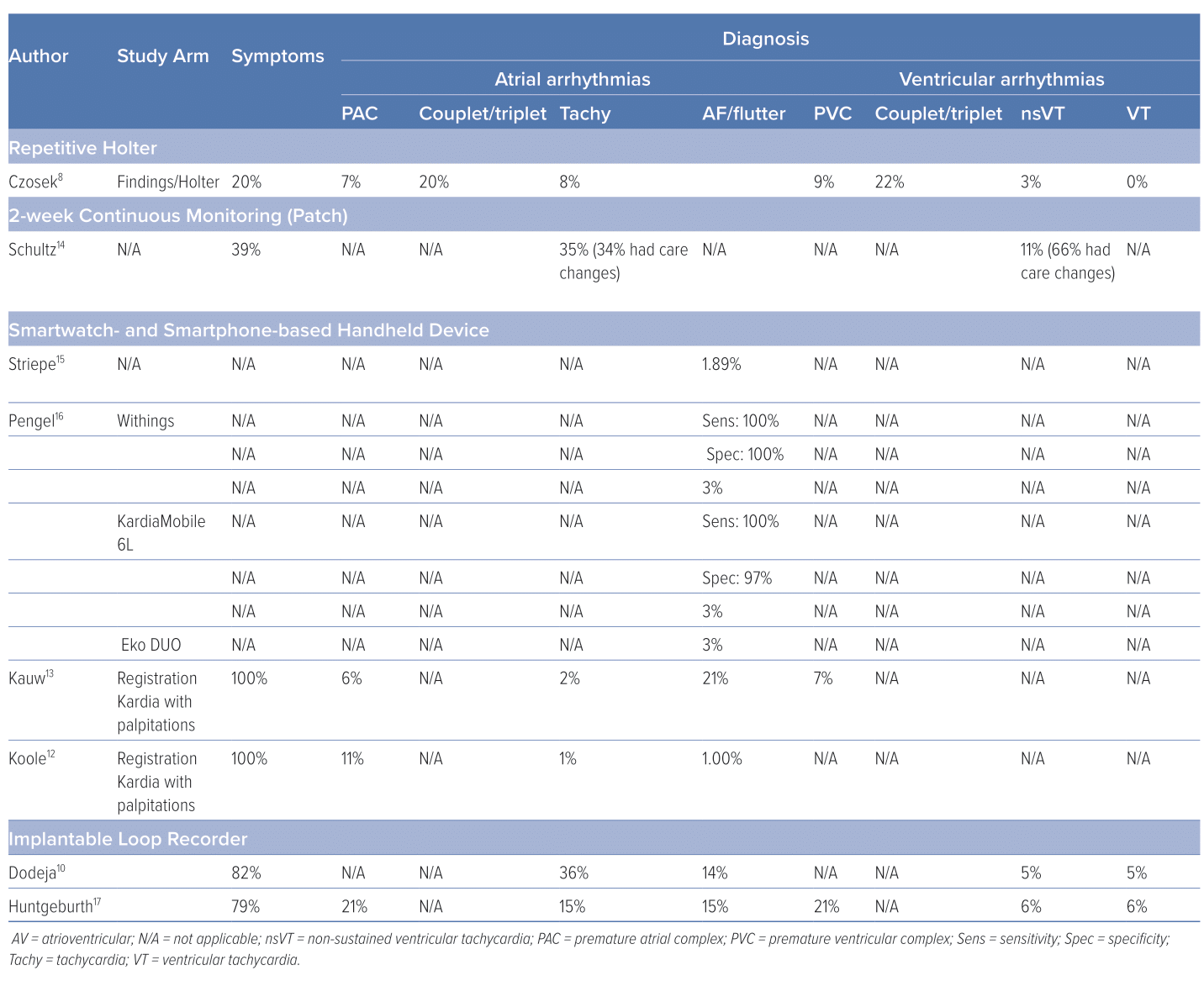
Cumulative Holter findings were defined as one or more Holter study from each individual patient for the detection of clinically significant arrhythmia by individual case history.8 Clinically significant arrhythmias and bradycardias were defined as any of the following: AF or flutter >30 seconds, ventricular tachycardia, non-sustained ventricular tachycardia, supraventricular tachycardia, sinus pauses of >3 seconds, second-degree type II (Mobitz II) atrioventricular block and third-degree atrioventricular block. The diagnostic yield was the primary outcome measure if reported, defined as the incidence of clinically relevant arrhythmia detected resulting in a change in patient management. These changes consisted of initiation or adjustment of medication, cardioversion, electrophysiology study, ablation or implantation of a cardiovascular implantable electronic device.10 The secondary outcome measure was the patient experience (net promoter score) and quality of life evaluated by using the patient-reported outcome measurement (PROM) questionnaires and other techniques.12,13 The quality and accuracy of the ECG were also evaluated if reported. The study characteristics are summarised in Table 1 and the study results are summarised in Table 2.
Cumulative Holter
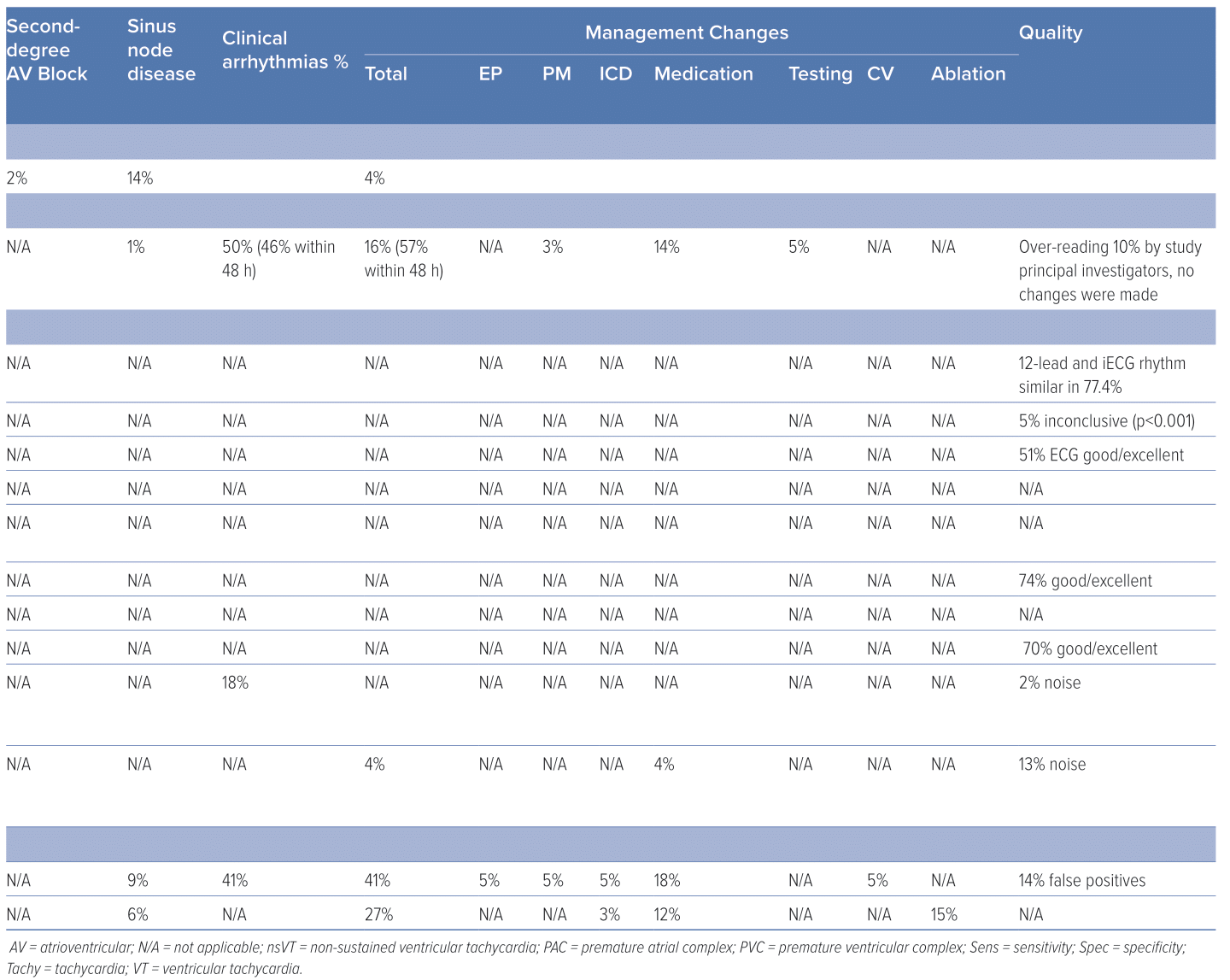
Czosek et al. evaluated 589 cumulative Holter screenings in 189 ACHD patients (TOF after surgical repair, dextro-transposition of the great arteries with previous atrial switch operation, including Mustard or Senning palliation and single ventricle physiology after Fontan palliation). The clinical utility and cost-effectiveness of consecutive Holter monitoring were evaluated. There were 22 (4%) Holter screenings that changed clinical management. However, 10 occurred after an earlier Holter with normal findings. A change of clinical management was defined as one or more of the following clinical events: initiation of an electrophysiological study, pacemaker or ICD implantation or replacement, initiation or change in antiarrhythmic drug therapy, or findings that directly led to additional diagnostics.
The opportunity of finding an arrhythmia causing a change in management increased with age. Patients aged 18–25 years undergoing a Holter received a change in management in 4% of Holter studies, while patients aged >25 years received a change in management in 6% of Holter studies. Furthermore, the underlying disease had an influence on the percentage of changes in management. After the Fontan procedure, the changes in management were 6%. Dextro-transposition of the great arteries resulted in changes in management in 5% of Holter studies.
The accuracy of a single Holter can be projected by the positive and negative predictive value. The individual Holter studies were analysed as separate events to determine the positive and negative predictive value of Holter monitoring in patients with and without clinical symptoms. The positive predictive value of patient symptoms predicting a clinically significant finding on Holter study was low, at 0.08, and the negative predictive value was 0.97.8
Two-week Continuous Monitoring
Schultz et. al. evaluated the diagnostic yield of 2-week continuous rhythm monitoring with an adhesive patch extended cardiac ambulatory monitoring (Zio monitor; iRhythm) in a retrospective cohort study of ACHD patients. The indications for extended monitoring included history of arrhythmia (20%), symptoms (39%), screening of arrhythmias due to history of CHD (28%), both symptoms and history of arrhythmia (10%), and abnormal testing (4%). The number and type of arrhythmias detected within and beyond the first 48 hours of monitoring were compared using Kaplan–Meier curves and Cox proportional hazards.14
In 50% of studies, the authors found clinically significant arrhythmia, of which 54% occurred after the first 48 hours. These findings led to a change in management, including pacemaker or ICD implantation, medication changes, or electrophysiology testing, in 16% of the total amount of 2-week continuous monitor studies. In 42%, this resulted from a finding that presented after 48 hours. Therefore, 7% of the total amount of 2-week continuous monitor studies contained an arrhythmia after 48 hours that resulted in a change in management. Whereas 34% of the supraventricular tachycardias resulted in a change in management, 66% of non-sustained ventricular tachycardias resulted in a change. Atrial and ventricular arrhythmia have been shown to occur in up to 43% of patients with TOF, and 100% of patients with Fontan physiology in long term follow-up. The accuracy of the 2-week continuous monitor was evaluated by over-reading 10% of the 2-week continuous monitor recordings by a study principal investigator. The authors concluded that no changes were indicated because of the good quality, and, therefore, no further over-reading was deemed necessary.14
Smartwatch- and Smartphone-based Single-lead ECG
Striepe et al. evaluated the accuracy of 30-second intelligent ECGs (iECG) of ACHD patients in three leads: Einthoven I/II/III-like leads.15 The aim of their study was to evaluate if the Apple Watch iECG is interpretable in patients with CHD, because a high-quality iECG would enable adult patients with CHD to use such a smartwatch as an event recorder. The iECGs were recorded by an Apple Watch Series 4. A total of 106 CHD patients were included.
The ECG parameters seemed to be independent of the patient’s characteristics, especially anatomy, electrical axis or situs. They were compared with a 12-channel ECG. A total of 77.4% of the iECGs diagnosed the same rhythm as by analysis by a cardiologist of the corresponding 12-lead ECG.15 However the Apple Watch cannot recognise an escape rhythm, pacemaker rhythm or VT due to lack of algorithms. The smartwatch can only display sinus rhythm, atrial fibrillation or inconclusive. Therefore, seven pacemaker rhythms were automatically diagnosed as sinus rhythms, nine sinus rhythms were recognised as unclassifiable and one sinus rhythm was defined as AF.
In a single-centre, prospective, cross-sectional study, Pengel et al. compared Withings ScanWatch, Eko DUO (precordial lead) and KardiaMobile 6L (6 leads) with the standard 12-lead ECG. They evaluated 30-s ECGs of ACHD patients acquired by the Withings ScanWatch in L lead.16 Of the ECGs acquired by the ScanWatch, 51% were either of good or excellent quality. A total of 70% of the Eko DUO device registrations and 74% of KardiaMobile 6L registrations were of good or excellent quality. The Withings ScanWatch algorithm was accurate for AF screening with a sensitivity of 100% and a specificity of 100%. Of these three devices, 54% of the patients preferred the Withings ScanWatch, 23% preferred the KardiaMobile 6L and 11% preferred Eko DUO. A majority of 80% was willing to use these devices.
Koole et al. studied 54 ACHD patients in a smartphone single-lead ECG program consisting of routine single-lead ECGs once a month and on demand in case of symptoms.12 Data were collected by mobile apps and matched with individualised thresholds. A management change by changing antiarrhythmic medication due to arrhythmias found by smartphone single-lead ECG was seen in 4% of patients. Of the recordings, 13% consisted of artefacts. The authors observed an overall positive effect on the quality of life of the ACHD patients who participated in an mHealth program.
An extension of the same smartphone single-lead ECG to 98 patients was evaluated by Kauw et al.13 The authors reported that clinically significant arrhythmia were found in 18% of patients. The majority of which were AF or flutter. They found four new diagnoses in this group (1 patient with sinus node dysfunction and 3 patients with paroxysmal AF), which led to intervention (electrical cardioversion) in two of these four cases.
Implantable Loop Recorders
ILRs, instruments for long-term rhythm monitoring, are used in a selected group of ACHD patients to detect arrhythmia, especially in cases of unexplained syncope, infrequent symptomatic palpitations and cryptogenic ischaemic stroke.
In a single-centre, retrospective review, Dodeja et al. evaluated the medical records of 22 ACHD patients who underwent an ILR implantation (Reveal LINQ; Medtronic) from 2014 to 2017.10 The ILR findings were with to the prior Holter/event monitors. The changes in management because of the ILR findings were also specified. In 41% of their patients (Fontan 32%, TOF 32%, other 36%) with ILR implantations, the authors found clinically significant arrhythmia that led to a change in patient management.10 This percentage was higher in the patient group with Fontan (57%) and lower in the patient group with TOF (14%). These changes in management consisted of medication changes (50%), ICD/pacemaker implantation (25%), cardioversion (12.5%) and electrophysiology studies (12.5%). Moreover, 14% of the ILR-registered arrhythmia were false positive.
Huntgeburth et al. performed a single-centre, retrospective, observational study in the German Heart Centre Munich.17 The ILR was implanted in 33 ACHD patients (mean age 43 ± 20 years; 42.4% women). During a mean observation period of 697 ± 433 days, clinically relevant arrhythmias, correlating with the patients’ complaints and symptoms, were detected in 19 patients (59.4%), of whom in nine patients (28.1%) the detected arrhythmia was considered an event requiring treatment. No acute complications were reported. Three out of 33 patients needed explanation of the device because of pain or wound dehiscence. The authors concluded that in symptomatic ACHD patients at risk of life-threatening cardiac events, ILR has a considerable complementary diagnostic value for the detection of malignant arrhythmias and in the differentiation from benign arrhythmias.
Discussion
Diagnostic Yield
The studies, although limited in number, on extended rhythm monitoring in ACHD patients were reviewed. The diagnostic yield of extended rhythm monitoring was high, and determined between 18% (using smartphone single lead ECG program) and 41% using ILR. Therefore, more relevant new diagnoses of arrhythmia can be found by extending continuous monitoring over a longer period.12–14 This finding is comparable to extended monitoring for AF in patients with cryptogenic stroke patients.18 In these patients, longer durations of monitoring were also associated with the highest diagnostic yield.19,20 Moreover, the optimal monitoring method and duration of monitoring in this population are also unclear.21–23 Solbiati et al. performed a systematic Cochrane review on ILR performance and concluded that available data are non-conclusive. The authors, therefore, recommended further research on ILR with clinically relevant outcomes.24 This could also be true for patients with ACHD.
Change in Clinical Care Management
The finding of an arrhythmia, important conduction disorder or severe bradycardia can cause change in clinical care management; for example, correction of underlying structural abnormalities, initiation or adjustment of medication, cardioversion, electrophysiology study, ablation, or implantation of a cardiovascular implantable electronic device. Schultz et al. showed detection of first arrhythmia that resulted in a change in management in 7% of ACHD patients only after 48 h of rhythm monitoring (average time of monitoring 9.5±3.1 days).14 Dodeja et al. demonstrated that ILR findings resulting in change in management were as high as 41%. Although these outcomes could not be compared directly due to differences in patient characteristics and indications for rhythm monitoring, they still emphasise the potency of extended rhythm screening beyond 48 h, as it could have a considerable effect on patient care. Clinically significant arrhythmia could be missed by a short period of rhythm monitoring.
In a Cochrane review, Solbiati et al. concluded that there is no evidence that an ILR-based diagnostic strategy reduces long-term mortality, as compared with a standard diagnostic assessment (very low-quality evidence). No data were available for short-term all-cause mortality. Moderate quality evidence shows that an ILR-based diagnostic strategy increased the rate of aetiological diagnosis as compared with a standard diagnostic pathway.24 Therefore, it is still debated if a change in clinical management has the ability to change clinically relevant outcomes, not only on mortality, but also on quality of life, syncope relapse and costs.
Device Accuracy
Our results demonstrate a high device accuracy of smartwatches and other ambulant ECG monitors, such as Zio patch, Eko DUO or KardiaMobile 6L in ACHD patients.16 Striepe et al. demonstrated that the Apple Watch can record reliable iECGs in patients with congenital heart disease of any type.15
Although false positive event recordings do occur and can lead to unnecessary stress for the patients and a higher workload for the healthcare workers, they probably outweigh the importance for the health of the ACHD patients in not missing clinically significant arrhythmia.
Limitations
This review was conducted to make an overview of the current arrhythmia screening instruments for ACHD patients, as new, innovative devices for this purpose are largely available nowadays. However, studies of ambulant extended screening of these patients for arrhythmia with new devices as an alternative to Holter monitoring or ILR are scarce. The outcomes and the manner of measuring the outcomes differed between the articles. The most prominent division was between articles that evaluated the changes in patient management resulting from their findings and the articles that evaluated the accuracy of their device. Therefore, it was difficult to compare the articles we reviewed. Most of the articles discussed in this review had a single-centre study design (six out of eight). This is a limitation, because it possibly degrades the transferability of the results. Also, two of the studies included are cross-sectional, which are less valuable, as it is not possible to evaluate changes over time. The sample size in all the studies was relatively small, because ACHD is not a common disease. In addition, most of the studies did not use a control group or used the patients as their own control group. Another limitation is the selection bias by indication, which could play an important role, as documented arrhythmia were one of the inclusion criteria in most of the articles. This could potentially imply that the patient population would be more prone to be admitted for arrhythmia in the last year before inclusion. In contrast, these patients could have received appropriate antiarrhythmic drug therapy leading to a reduction in subsequent changes in management during the study period.2 There is also a risk of bias because of risk profile. ILR is implanted in highly selected ACHD patients with a high-risk profile, potentially accounting for a higher diagnostic yield. Furthermore, for this review, only one database was used, namely PubMed, and a restriction was put on language, which possibly resulted in the overlooking of relevant articles from other databases or languages. In addition, a restriction was put on publication date; older relevant articles may have been missed.
Future Directions
mHealth, defined as ambulant diagnostics, as wearables, mobile health applications (apps), patches with sensors and smartphone possibilities, are rapidly evolving, and increasing in number.13,25,26 mHealth can be an alternative for Holter monitoring and ILR monitoring. It has the advantage of the possibility of, almost continuously, monitoring heart rhythm for a patient’s lifetime, whereas Holter monitoring is limited to approximately 48 hours and ILR monitoring is limited by the lifespan of the implanted battery.
However, to implement mHealth in routine care of ACHD patients is a challenge. First, patients and physicians should be convinced of the added value using mHealth. Second, these solutions should include a critical assessment of the associated costs.27 The financial implications of mHealth include initial setup and maintenance expenses, and the potential long-term economic benefits. Insight into these costs is pivotal for stakeholders to make robust decisions, and ensure the sustainable and equitable deployment of mHealth technologies. To be affordable, data handling should be optimised and automated. Furthermore, costs are dependent of the number of participants of a specific mHealth program, because upfront investment costs might be considerable, fixed and variable costs change very little per additional patient compared with usual care.28 Costs might be different between healthcare systems and countries. More research is needed to analyse the cost-effectiveness of mHealth solutions regarding these aspects.
Although health system governance, reimbursement and technological factors may complicate implementation, tools to identify barriers to implementing digital health and recommendations for overcoming them are increasingly available.29–32
The optimal mode and duration of screening for arrhythmia can be different for asymptomatic patients compared with those with palpitations or who have experienced sudden syncope.33 Therefore, new research initiatives for extended screening for arrhythmias in ACHD patients are needed to reveal whether a reduction in morbidity and mortality can be achieved in a specific patient population with early event recognition and intervention.
Conclusion
A limited number of studies on rhythm monitoring in ACHD patients demonstrated a higher rate of arrhythmia and bradycardia detection, leading to clinical care changes by extending the time of rhythm monitoring to >24 hours. These clinical care changes include medication optimisation, cardioversion, electrophysiology study, ablation or implantation of a cardiovascular implantable electronic device. Therefore, extended monitoring seems to be important for patient care. Cumulative Holter monitoring, monitoring by wearable patches, smartwatches and smartphone single-lead ECGs are, in symptomatic patients, a good alternative to ILRs. However, the optimal mode of detection is still unclear due to the lack of head-to-head comparisons. These findings emphasise that randomised studies are needed to determine the efficacy and indications for the various available instruments in ACHD patients.
To our knowledge, this is the first review on all available rhythm detection devices in ACHD patients to perform extended screening for arrhythmia. Our findings provide a broad insight in the device accuracy, diagnostic yield and clinical outcome. 
Clinical Perspective
- Extended arrhythmia monitoring in adult congenital heart disease patients has a high diagnostic yield.
- Nowadays, a variety of non-invasive tools are available for ambulant continuous heart rhythm monitoring.
- We need more studies for choosing the best available option for extended heart rhythm monitoring in adult congenital heart disease patients.