AF is the most common arrhythmia and about 10% of the general population above the age of 65 years are affected by this condition.1 The mortality and morbidity of AF is well established, with a higher risk of stroke and heart failure in older patients with comorbidities.2 The pathophysiology of AF is complex and variable, making its management extremely challenging. Epidemiological studies have identified a vast array of risk factors that account for its high prevalence and the difficult implementation of primary prevention measures.3–7 Electrophysiological mapping studies over several decades have also provided us with invaluable insights. The seminal finding of spontaneous initiation of arrhythmia by ectopic focal activity in the pulmonary veins revolutionised treatment of AF; however, it is clear from recent studies that the electrophysiological substrates involved are far more heterogeneous.8
Conventional treatment for AF has focused on rate and rhythm control using anti-arrhythmic drugs (AADs), as well as anticoagulation therapy based on individual risk profile.9 Treatment can be challenging when using medical therapy alone due to a lack of atrial specific agents, modest efficacy and significant toxicities of AADs. Catheter ablation of AF is a well-established treatment for patients in which sinus rhythm is desired, such as those with refractory symptoms despite maximal medical therapy, heart failure secondary to AF and intolerance to AADs. Radiofrequency catheter ablation (RFA) of the pulmonary veins was the first and most widely performed ablation procedure and cryoablation is a newer and rapidly progressing technique which has resulted in shorter procedure times and reduced treatment costs.10
Safety and success rates of catheter ablation have improved. However, although outcomes are generally regarded as good in cases of paroxysmal AF (PAF defined by <7 days of continuous AF) and, in recent times, persistent AF (Pe-AF defined as >7 days AF but continuous duration <1 year), there remains a need for a more robust procedure for patients with long-standing persistent AF (LS Pe-AF defined as continuous AF >1 year).11
Convergent ablation – also known as the convergent procedure – is a hybrid technique combining an endocardial RF ablation procedure with minimally invasive epicardial surgical ablation of the posterior left atrial (LA) wall. This procedure targets the posterior wall of the LA, an area difficult to ablate effectively using a catheter-based approach. Studies have shown that in patients with longer AF durations, atrial stretch leads to structural and electrical atrial remodelling and development of vulnerable atrial substrate, particularly in the posterior LA.12 The rationale behind the convergent procedure is to target this substrate in combination with conventional endocardial pulmonary vein isolation.
Significant interest in hybrid ablation began about 10 years ago. Although there have been no randomised clinical trials, several observational studies, largely from single centres, have been published describing the results of this relatively new strategy as a feasible and effective treatment approach. Some suggest that hybrid ablation may be more effective than lone endocardial ablation in achieving the highly elusive goal of maintaining sinus rhythm in patients with non-paroxysmal AF.
In this article, we will review the safety and efficacy of the hybrid approach, as well as the role of the electrophysiologist in convergent ablation.
Pathophysiology of AF
Initiation and progression of AF requires vulnerable atrial substrate, with the formation of this substrate dependant upon a host of both modifiable and non-modifiable risk factors. Population-based studies have revealed increasing age, diabetes, hypertension, valvular heart disease, obesity and obstructive sleep apnoea as risk factors contributing to AF.7 The primary pathological change induced by these various diseases appears to be a final common pathway of atrial cardiomyopathy, a complex of structural, architectural, contractile and electrophysiological changes, characterised mainly by atrial dilatation and fibrosis.13
The electrophysiological mechanisms underpinning the initiation and maintenance of AF are still subject to ongoing debate. Seminal research by Haïssaguerre et al. in 1998, reported focal ectopic firing arising from the myocardial sleeves in the pulmonary veins in patients with paroxysmal AF, with ablation of these foci reducing the arrhythmia burden.8 Membrane instability due to abnormal calcium handling, complex fibre architecture and re-entry mechanisms all contribute to this abnormal ectopic activity.14
Where pulmonary vein firing has been identified as the trigger for initiation of AF, in recent years the importance of vulnerable atrial substrate and re-entry has been identified.15 Re-entry is stabilised by anatomical and electrophysiological atrial abnormalities, promoting maintenance of arrhythmia, particularly in patients with non-paroxysmal AF. There are three dominant hypotheses regarding the mechanism of the maintenance of AF: multiple independent wavelets, re-entrant rotors and the double-layer hypothesis.
The multiple wavelet hypothesis was first described by Moe et al. in 1959, who postulated that the disorganised activity of AF may be due to the random propagation of multiple independent wavelets in a medium of dispersed refractoriness.16 Experimental evidence for this was provided by Allesie et al. in a dog model of AF, where several independent wavelets propagated through both atria.17 This theory was the basis of the surgical maze procedure, where the atria are compartmentalised to prevent propagation of wavelets.18
In recent years, the presence of stable spiral areas of functional re-entry within the atria of patients in AF, known as ‘rotors’, have been reported. The conventional ablation for AF with or without focal impulse and Rotor Modulation (CONFIRM) trial provided clinical evidence for this theory in 2012, when it reported that rotors were mapped in 97% of 101 patients with sustained AF, and that ablation of these rotors (focal impulse and rotor modulation – FIRM) improved outcomes of AF ablation.19 However, these results have not been consistently replicated in other mapping studies.20 It is postulated that due to the low resolution of the 64-pole basket mapping catheters used in the CONFIRM study, the visualised rotor activity may have been either artefactual, or represented other forms of re-entry. In addition, the CONFIRM trial mapped patients with pacing-induced AF, which may be mechanistically different from chronic AF.
In contrast to the above hypothesis, Allessie et al. demonstrated no evidence of rotor activity with simultaneous high-density endo-epicardial mapping of patients with AF undergoing cardiac surgery.20 Fibrillation maps showed complex and continuously changing activation patterns by a large number of narrow fibrillation waves, separated by lines of longitudinal conduction block. They postulated that instead of AF being maintained by stable sources such as rotors, AF is perpetuated by two electrically dissociated layers of narrow wavelets which ‘feed’ each other via constant endo-epicardial breakthrough. The frequency of this breakthrough was seen to be four times higher in subjects with long-standing persistent AF compared with those with acute AF, possibly explaining the poor outcomes for conventional endocardial catheter ablation in this group of patients.
Therefore, it is evident that the pathophysiology of AF is highly complex and not yet fully understood. This makes its management extremely challenging.
Catheter Ablation
With AF being such an electrophysiologically heterogeneous entity, it is no wonder that strategies for ablation are continually developing as the search continues for optimal treatments, especially for patients with LS Pe-AF. Catheter ablation for AF was first described in 1994, and these procedures included ablation of right atrial triggering mechanisms, creation of right atrial linear lesions and attempting to recreate a surgical maze of lesions.21,22 However, these methods had limited success. The seminal finding of pulmonary vein trigger sites led to the contemporary era of pulmonary vein isolation (PVI) for AF.8
The field of PVI is constantly developing. The most widely performed procedure is RF wide antral circumferential ablation.23 With advancements in catheter technologies, including the use of second-generation irrigated catheters and contact-force sensing catheters, the success rates of RF ablation in PAF has been reported to be about 75.7% (162 of 214 patients).24 In recent years, the advent of cryoballoon technology has shown promise with a view to reducing procedural time. The FIRE AND ICE: Comparative study of two ablation procedures in patients with atrial fibrillation randomised control trial reported that cryoballoon ablation was non-inferior to RF ablation with respect to efficacy in treatment of patients with drug-refractory AF, with no significant difference between the methods with regards to overall safety.25
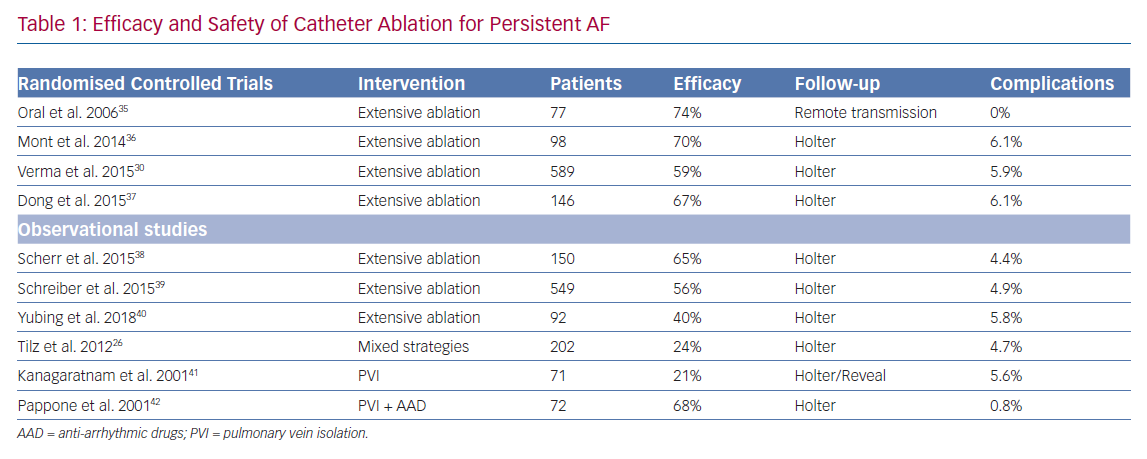
While success rates are generally regarded as good for PAF and Pe-AF, this is not the case in the population of patients who have been in continuous AF for more than 1 year, defined as LS Pe-AF (Table 1). Data from the Hamburg Sequential Ablation Strategy reported that during 5-year follow-up of 202 patients who underwent a sequential ablation strategy for symptomatic LS Pe-AF, the single procedure success rate was 20%, rising to 45% with multiple procedures.26
Much effort has been invested in non-PVI forms of ablation, such as alternative trigger ablation and substrate modification. Reports of higher level of vulnerable atrial substrate in patients with LS Pe-AF has led researchers to hypothesise that extensive atrial ablation is superior to PVI alone for this population.27 Studies have shown that linear atrial ablation lines have improved arrhythmia-free survival in patients with Pe-AF, without increased complications.28 There has also been a great deal of interest in ablation of complex fractionated electrograms (CFAE), postulated to be areas of continuous re-entry of fibrillation waves into the same area, or overlapping of different wavelets entering the same area at different times. Nadamanee et al.. reported a 91% arrhythmia-free survival rate in a cohort of 121 patients with either PAF or Pe-AF undergoing CFAE ablation without PVI.29
However, the results from these smaller studies were not reproduced in the Substrate and Trigger Ablation for Reduction in AF Trial II (STAR AF II).30 This multicentre randomised clinical trial assigned patients in a 1:4:4 ratio to: PVI alone; PVI plus CFAE ablation; and PVI plus linear ablation of the LA. There was no reduction in the rate of recurrence of AF when either CFAE ablation or linear ablation was added to PVI. The procedure time and serious complication rate was higher in the two groups undergoing more extensive ablation.
Catheter-based posterior wall isolation (PWI) has also been examined extensively. A non-randomised study by Aryana et al. demonstrated superiority of PVI plus PWI compared with lone PVI using a combination of cryoballoon and RFA in patients with Pe-AF.31 A systematic review of 17 studies by Thiyagarajah et al. demonstrated good outcomes for catheter-based PWI with a 12-month freedom from AF of 61.9%.32 However, randomised controlled trials comparing PWI with PVI (three studies; n=444) yielded conflicting results and could not confirm an incremental benefit to PWI.
It remains unclear why there is a lack of benefit from additional ablation. One explanation is that there is significant intra-procedural heterogeneity between studies and centres when performing extensive ablation, based on operator experience. Catheter-based technology, while advancing rapidly, is still an imperfect tool to create continuous linear ablation lines, and this deficiency can leave pro-arrhythmic gaps which manifest clinically as recurrent atrial tachycardias.33 In addition, the concern of damaging the phrenic nerve, lungs or oesophagus hamper the ability to create transmural lesions with endocardial ablation, especially on the posterior wall of the LA.34 Many believe that a more robust, reproducible method of ablation will be required to treat this difficult group of patients with LS-Pe AF.
Convergent Ablation
In recent years, convergent ablation – a hybrid endocardial and epicardial ablation approach – has emerged as a novel approach to treating LS-Pe AF.43 Surgical AF ablation was first described in 1987. The Cox-maze procedure involved creating linear incisions in the atrial walls which created a block against propagating wavelets and macro re-entrant circuits.44 While the procedure was efficacious, it resulted in high rates of chronotropic incompetence and pacemaker implantation. The procedure was updated to the maze II and then the maze III procedure, which used modified incisions in an attempt to maintain sinus node function. The most modern iteration, the Cox-maze IV, uses ablation rather than surgical incisions. The procedural complexity of the maze procedures, as well as the need for median sternotomy and cardiopulmonary bypass means that it has been superseded by catheter ablation. However, it did have the advantages of creating continuous linear transmural lesions under direct vision, with reduced risk of damaging abutting organs.
In recent years, studies have been conducted into the totally thoracoscopic maze procedure (TT-maze), which involves bilateral video-assisted thoracoscopic (VATS) access, and uses RF ablation to perform pulmonary vein isolation and an LA posterior box lesion.45 This procedure has shown promise but it is lengthy, extremely technically challenging and requires bilateral lung deflation.
The convergent procedure was developed as a multidisciplinary two-stage approach to AF ablation, involving both cardiac electrophysiologists and cardiac surgical teams. The first procedure involves closed-chest access via a trans-diaphragmatic pericardial window, followed by epicardial ablation of the posterior LA. The trans-diaphragmatic approach has since been abandoned in favour of a sub-xiphoid approach. This ablation electrically silences the posterior LA, aiming to interrupt all known AF substrates. This is followed by an endocardial catheter ablation, either at the same sitting or at a later date. During this procedure, electrical pulmonary vein isolation is confirmed, the results of the posterior wall ablation are checked and fine-tuned if necessary. The lesion set is completed by performing endocardial ablation of structures which cannot be accessed epicardially due to pericardial reflections.
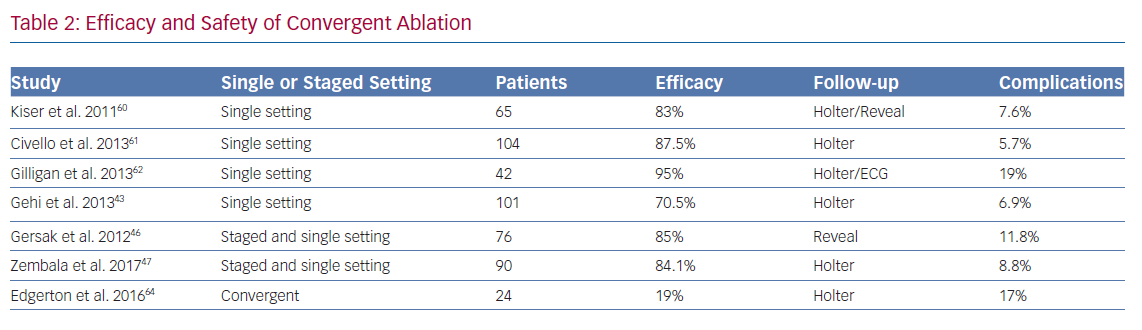
As yet, no data from randomised controlled trials have been published comparing convergent procedure with standard catheter ablation. Data has come from observational studies (Table 2) and meta-analyses. A multicentre study by Gersak et al. followed up 50 consecutive patients, 70% with LS Pe-AF, undergoing convergent ablation.46 It showed that 81% of the patients had less than 3% AF burden at 12 months. There was a 4% mortality in this study as two patients had fatal atrio-oesophageal fistulas.
A study by Zembala et al. (n=90; LS Pe-AF=51) revealed similar results, with 86% of patients followed up at one year remaining in sinus rhythm and 62% being off AADs.47 This group reported three serious complications (death of unknown cause, major surgical bleeding and tamponade) in the first 27 patients, and one in the remaining 63. The overall serious adverse event rate was 4.5%. Luo et al. performed a meta-analysis of six observational studies examining the convergent procedure (n=478), which reported sinus rhythm maintenance at 12 months to be 84%, but this reduced to 60.2% after two studies were trimmed according to the trim-and-fill method.48 This reported a serious complication rate of 9% and a mortality rate of 1.7%.
Recent meta-analyses have also been carried out assessing the efficacy and safety of hybrid ablation with mixed results.49,50 However, these studies used the previously non-standardised definition of hybrid ablation and included patients undergoing thoracoscopic epicardial ablation. As such, the results from using convergent ablation alone cannot be extrapolated from such meta-analyses. This non-standardised definition of hybrid ablation is a limitation of existing literature outcomes.
It is evident that there is considerable variability in reported efficacy and safety outcomes of convergent ablation. This probably represents the heterogeneity of interventions being analysed in observational studies.51
There has been an evolution of the surgical technique to a sub-xiphoid approach rather than trans-diaphragmatic, as well as improvements in surgical ablation equipment. This explains higher initial complication rates to some degree. Outcomes of patients undergoing convergent ablation in a single setting and a staged setting tend to be grouped together for analysis and as yet there is no clear evidence of superiority for either method compared with the other. The advantage to a single-setting procedure is a single hospital visit and immediate endocardial mapping to confirm integrity of epicardial lesions. The caveat to this is that peri-myocyte oedema following epicardial ablation can hinder endocardial electrophysiological assessment.52 A formal comparison between the two methods will need to be undertaken to elucidate any differences in performance.
The heterogeneity of existing data means that the jury is still out on convergent ablation. Randomised controlled trials with reproducible methodology and clear efficacy endpoints are needed to examine whether the procedure is superior to catheter ablation in certain patient groups. The Epi/Endo Ablation for Treatment of Persistent AF (CONVERGE; NCT01984346) trial has finished recruitment, and will hopefully answer some of these questions.
The next stage in the evolution of convergent ablation may be electrical isolation of the left atrial appendage (LAA) as an adjunctive procedure, as this structure has been implicated in refractory AF.53 Potential benefits of catheter ablation of the LAA have been demonstrated, but this procedure is time-consuming and not widely performed.54 There is also ongoing concern regarding increased stroke risk caused by electromechanical dissociation of the post-ablation LAA leading to stasis.55 Surgical LAA exclusion or ligation at the time of open heart surgery has been in practice for several decades and retrospective studies have suggested this reduces stroke risk in patients with AF.56 Other established methods of LAA closure for stroke prevention include the Watchman (Boston Scientific) and Lariat (Willis-Knighton) devices, but a complete examination of this subject is beyond the scope of this review.
There has been interest in combining the convergent procedure with a thoracoscopically delivered epicardial closure device known as the Atriclip (AtriCure). Data on this combined procedure is limited to case series.57 Outcomes from using the Atriclip combined with open heart surgery or thoracoscopic ablation have been promising. A systematic review of 922 patients reported a rate of ischaemic stroke at between 0.2–1.5 per 100 patient years, significantly less than the predicted rate in this cohort of 2.9 per 100 patient years.58
There is a paucity of evidence assessing the effect of Atriclip on long-term arrhythmia burden. However, in a case series of patients undergoing coronary artery bypass grafting (CABG), Atriclip has been shown to provide immediate electrical LAA isolation confirmed with bidirectional block during pacing manoeuvres.59 Given the potential complications of combining Atriclip with convergent ablation, including exposing patients to single-lung ventilation in a procedure that would otherwise not require this, it is crucial that clinical trials are performed to examine its additional efficacy benefit before recommending this as standard practice.
The Role of the Electrophysiologist in Convergent Ablation
Convergent ablation is a truly multidisciplinary approach to the treatment of AF. The AF heart team consists of an electrophysiologist, cardiac surgeons, specialist nurses and physiologists. The electrophysiologist is the central coordinator of this process, as they are the professional with the most experience and knowledge at managing this complex arrhythmia.
Electrophysiologists play a crucial role, not only in performing the endocardial stage of the procedure, but somewhat more importantly in: establishing the service; developing safe and effective pathways and protocols; and patient selection.
Catheter Ablation and Convergent Ablation
The addition of catheter ablation to epicardial ablation transforms an anatomical procedure into an electrophysiological one. When performing a staged endocardial mapping and ablation, the electrophysiologist is able to check for the electrical isolation provided by the surgical lesion set, and ‘fill in the gaps’ where required, including in areas that are epicardially inaccessible beneath pericardial reflections.64 The majority of these procedures are being performed with RF ablation, however some centres are now performing cryoballoon ablation – the so-called ‘cryoconvergent’ procedure.65
Theoretically this hybrid approach has the benefits of a more robust lesion set, however there are still concerns about whether the added benefit of the catheter ablation outweighs the exposure to peri-procedural complications. A recent meta-analysis reported that isolated epicardial ablation was equally efficacious to hybrid ablation (either convergent or thoracoscopic) in providing freedom from AF, with increased safety rates.49 It should be noted, however, that there were a higher proportion of patients with LS-Pe AF and increased atrial dimensions among the patients undergoing hybrid ablation in this analysis. Randomised controlled trials of patients with LS-Pe AF are needed to more reliably assess the efficacy and safety of convergent ablation compared with isolated epicardial ablation.
Establishing the Convergent Ablation Service
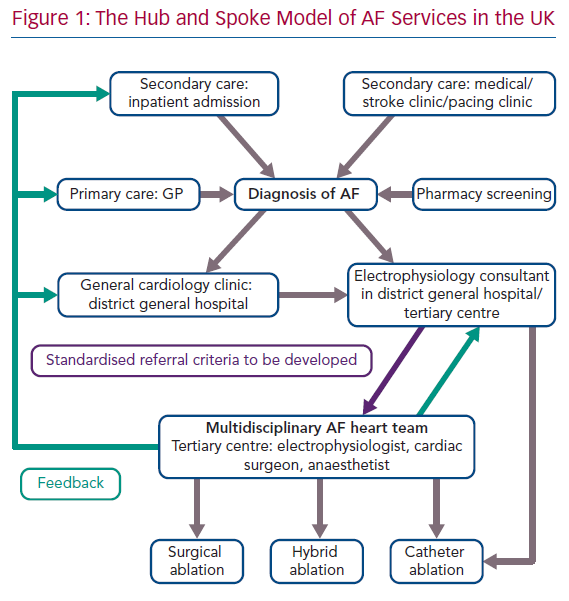
In the UK, the majority of electrophysiology services follow a hub and spoke model with most consultant electrophysiologists having cross-site roles at both a tertiary or quaternary centre, and a district general hospital to provide outreach specialty expertise (Figure 1).66 The convergent ablation service, much like conventional ablation, follows this model, with the majority of patients having first contact at their local hospitals, followed by referral to a major cardiac hub for the procedure.67
This form of cross-site, cross-specialty collaboration is already firmly rooted in other areas of cardiology, with joint cardiology and cardiothoracic meetings taking place in most hospitals to discuss treatments for coronary revascularisation and structural heart disease.68 The advent of the convergent procedure marks the first time that arrhythmia management will require this form of multidisciplinary decision-making.69 Electrophysiologists are crucial to the coordination of this process, not only by establishing and facilitating this type of collaboration, but also by ensuring that information and education about the procedure is disseminated to general cardiologists, who will often have appropriate patients for this treatment under their care on the ward and in clinic.
Patient Selection
Some of the challenges inherent in convergent ablation include the additional potential risks from performing a second procedure in a staged approach, the additional costs of a second procedure, and the potential need for a longer or a further stay in hospital. It is therefore critical to select the patients who would benefit most from upfront hybrid ablation as opposed to catheter ablation. Electrophysiologists are vital to this selection and decision-making process, having in-depth knowledge of AF treatments, including medical management, conventional catheter ablation or pacing plus atrioventricular node ablation.
Theoretically, the patients who would benefit most from convergent ablation are those who are predicted to have poor outcomes from conventional catheter ablation. We know that duration of AF is a significant factor. Advances in radiofrequency catheter and cryoballoon technology have increased success rates in patients with PAF, and outcomes are also improving for patients with Pe-AF.25,70 Conventional ablation in patients with LS-Pe-AF currently has very poor efficacy with a single procedure.26
LA size and function is also examined closely prior to catheter ablation, with conventional theories stating that increased LA dimensions by conventional echo criteria are predictive of poor procedural efficacy outcomes.71 Observational studies and meta-analyses examining this relationship, however, have shown mixed results.72 High burden of LA structural remodelling on cardiac MRI has been demonstrated to correlate with recurrence of AF after catheter ablation, but this technology and expertise is not widely available.73 A recent meta-analysis suggested that reduction in LA strain measurement by 2D speckle tracking echocardiography may be superior to increased volumetric LA measurements in predicting long-term failure of catheter ablation.72 Further studies are required to determine whether convergent ablation is more successful in these patients.
The role of convergent ablation in patients with previous failed catheter ablations has not yet been elucidated. Several observational studies included patients with previous ablations as part of their cohorts but differences in outcomes between these patients and those undergoing hybrid ablation as their first procedure have not been formally examined.47 CONVERGE-IDE recruited only de novo patients as part of the inclusion criteria. There remains scope, therefore, for future trials assessing convergent versus repeat catheter ablation in patients with AF recurrence after one procedure.
Comparative cost-effectiveness studies need to be performed to determine the feasibility of convergent as a widespread ablation strategy. Using a Markov micro-simulation model, Anderson et al. concluded that convergent ablation results in superior maintenance of sinus rhythm with fewer repeat procedures compared with catheter ablation, leading to lower cost and higher quality-adjusted life-years after 5 years.74 This study was limited by the use of observational data to predict the efficacy of convergent ablation, and repeat cost-analyses should be performed after publication of clinical trial data.
Clinical practice with regards to patient selection for convergent ablation is highly variable worldwide. There are no standardised referral criteria for hybrid ablation even within centres, and these decisions are made based on consultant preferences. Our own practice considers convergent ablation for patients with LS-PeAF and increased LA diameter, but these criteria will need to be refined as the available data expands. After publication of randomised clinical trials, cost-analyses and further examination of predictive factors for failure of catheter ablation, we would advocate development of a scoring system to determine which patients may benefit from a hybrid approach. All patients considered for ablation who meet this threshold should be discussed with the AF heart team, as the combined expertise of electrophysiologists, surgeons and anaesthetists are needed to make these challenging and important decisions, after detailed discussions with the patient.
Pathway and Protocol Development
Developing pathways for a hybrid procedure to ensure quality care and patient safety is a challenging and meticulous endeavour. The patient journey from first contact to procedure and subsequent recovery is well established in other areas of surgery and cardiology, but these models need to be transposed onto the provision of convergent ablation.75
Coordinating a two-stage procedure adds multiple layers of complexity to service protocol and patient flow. It is important that the electrophysiologist recognises this and works in collaboration with administrative and nursing staff to create a clear pathway. This involves publication of rigorous pre-assessment guidelines for convergent ablation, and liaising with anaesthetists and cardiology/cardiothoracic nursing staff regarding additional checklists and safety protocols.52,76 It involves clear communication with the bookings team and bed managers, as an overnight stay is required after the epicardial ablation. Given that convergent is a relatively novel procedure, appropriate training needs to be arranged for theatre and ward staff regarding peri- and post-procedural management, including common complications and complex pain needs which professionals in certain specialties may not be accustomed to treating.
An area which is often neglected is rigorous follow-up and rehabilitation. Referral for cardiac rehabilitation are routine in pathways after procedures such as PCI, CABG and trans-catheter aortic valve intervention.78 Currently, this is not the case after conventional ablation. However, given that convergent ablation involves two invasive procedures, requiring at least one if not two episodes of general anaesthesia in a relatively comorbid population, it is vital that patients are educated thoroughly on the importance of exercise and risk factor modification for general cardiovascular health, as well as individualised risk-based referral to cardiac rehabilitation classes. As the clinicians ultimately responsible for the management of these patients, it is important that electrophysiologists take the lead in this holistic approach to patient care.
Conclusion
Convergent ablation has shown some promise as an efficacious treatment in patients with LS-PeAF. With so much uncertainty regarding the electrophysiological mechanisms of AF, convergent ablation provides a way to reliably perform reproducible linear lesions of the posterior left atrium, thus targeting all known substrates including rotors, wavelets and epicardial breakthrough.
Current observational studies have provided mixed results, but as the volume and operator experience of this procedure increases, it is possible that when this procedure is perfected it will provide the best outcomes in a group of patients with LS Pe-AF who traditionally have poor results from conventional ablation. Randomised controlled trials will determine if this is the case.
The electrophysiologist’s role in convergent ablation includes technical operator, manager, coordinator, leader and spokesperson in introducing this novel procedure as a safe, cost-effective, deliverable service. The success of convergent ablation will not only depend upon its efficacy and safety determined by clinical trials, but by the ability of electrophysiologists to be the glue that holds together a vast multidisciplinary team, and make the vision of widespread availability of convergent ablation a reality.
Clinical Perspective
- Convergent ablation shows promise in improving the treatment of long-standing persistent AF, where conventional catheter ablation has generally poor efficacy outcomes. Data thus far has come from observational studies. The first randomised controlled trial, CONVERGE-IDE, has finished recruitment and results are awaited.
- Convergent ablation is a truly multidisciplinary collaborative electrophysiology service. The electrophysiologist plays a crucial role, in particular with optimum patient selection, pathway development and team coordination.
- Standardised criteria need to be developed for referral to the AF heart team to discuss complex patients who may benefit from alternative ablation strategies.