Hypertrophic cardiomyopathy (HCM) is a common genetic cardiac disorder, with an autosomal dominant mechanism of inheritance.1,2 It has a prevalence of 1 in 500 within the general population, and is a known cause of sudden cardiac death.2,3 Recognised autosomal dominant mutations within sarcomere proteins are found in 55 % of adolescents with sporadic HCM.4 Characteristic echocardiographic features are well described;2 a left ventricular (LV) wall thickness ≥15 mm not explained by loading conditions is considered diagnostic for HCM, but diagnostic challenges exist.5 Co-existent pathologies associated with increased cardiac load can make ascertainment of the causative pathway of LV hypertrophy difficult.6 In addition, diagnosis in the late disease phase can be confused by ventricular dilatation associated with LV wall thinning.7
AF is the most common sustained arrhythmia,8 and is associated with a significantly increased risk of stroke and heart failure.9 HCM has been associated with the development of both AF and thromboembolic events.5 Indeed, 48-hour ambulatory monitoring is advised as part of the initial HCM assessment, in part, to establish whether atrial tachyarrhythmias are present.5 Atrial fibrosis has been demonstrated in some individuals with HCM, but an atrial histology similar to the HCM ventricular pathology has not been demonstrated.10 Despite the common nature of both conditions, and their considerable overlap, the role of anticoagulation in this population has not been fully investigated. This review aims to assess the evidence surrounding the development of thromboembolism in patients with HCM and AF.
Hypertrophic Cardiomyopathy and the Development of Atrial Fibrillation
Although AF is common in patients with HCM, prevalence rates differ significantly between studies; prevalence has been described to be between 12 and 28 %.11–18 Eriksson et al. showed that AF developed in 12 % of patients (13/105) over a mean follow-up period of 13.6 ± 8.3 years.13 Furthermore, they found that AF was the initial disease presentation in 10 % of patients (10/105). This report of a retrospective cohort analysis does not clearly detail how AF was determined. As such, the authors may have underestimated the true prevalence of AF in this population. In a retrospective cohort (n=4,821), Guttmann et al. demonstrated an AF prevalence of 12.5 % at baseline.19
The reported prevalence found in cohorts evaluated at specialist HCM centres has been found to be significantly higher. Binder and colleagues reported an AF prevalence of 28 % in patients with apical HCM.11 This rate is supported by other registries.12,16,20 A systematic review examining AF in the HCM population included 7,381 patients in the analysis. The overall prevalence of AF in this population was 22.5 % (95% CI [20.1–24.8]).21 However, it should be noted that not all reports were included in the systematic review, including some citing lower prevalence levels. The authors also highlighted difficulties with the analysis due to heterogeneity of the study populations.
Kawasaki et al. undertook prospective 24-hour Holter monitoring on patients with HCM, where those with pre-existing AF had been excluded.14 They demonstrated that 3 % of patients were shown to have AF paroxysms lasting >30 seconds.
AF has been shown to be subclinical in a substantial proportion of the general population,22 this has led to concern that a similar proportion of patients with HCM and AF may be under-recognised. Robinson et al. demonstrated that in a cohort of 52 consecutive patients with HCM developing AF, 89 % had a change in symptoms with the onset of the arrhythmia.23 Similar numbers have been reported by other groups.17 In a small cohort (n=44) of patients with HCM undergoing device implantation (implantable cardioverter defibrillator [ICD], permanent pacemaker, or loop recorder), in those developing de novo AF (n=16) 88 % were asymptomatic.24
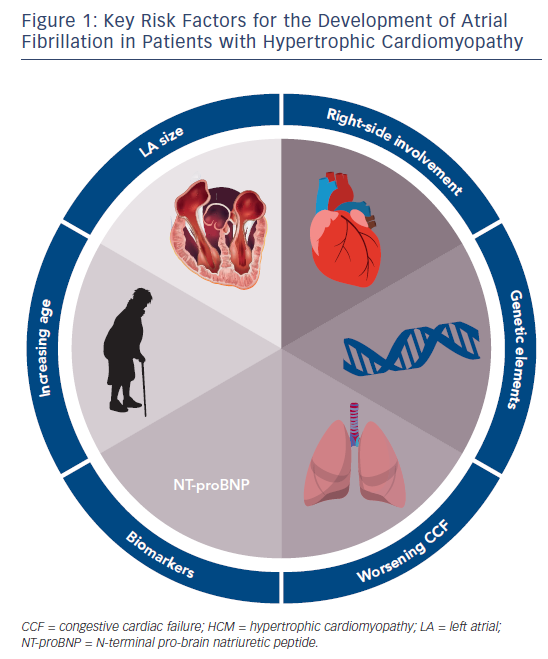
Risk Factors For the Presence of Atrial Fibrillation in Hypertrophic Cardiomyopathy
Several risk factors for the development of AF in patients with HCM have been identified. N-terminal pro-brain natriuretic peptide (NT-proBNP) levels have been shown to positively correlate with the presence of AF at baseline.12 Prevalent AF was seen in 11 % (7 patients) in the lowest tertile of NT-proBNP levels compared with 36 % (22 patients) in the highest tertile. Retrospective analysis of a large, single-centre cohort confirmed that BNP levels are increased in patients with HCM and AF;25 this is in line with evidence supporting a significant prognostic role of NT-proBNP in predicting the development of AF.26,27
Several studies have reported an association between left atrial (LA) size and the presence of AF.28,29 Spirito et al. examined a consecutive cohort of 668 low-risk patients with HCM (no major sudden death risk factors, New York Heart Association [NYHA] class I or II and no history of AF).28 Over a median follow-up of 5.3 years, the development of AF was associated with increased baseline LA diameter with a relative risk of 4.65 (95 % CI [2.18–9.92]) in patients with an LA diameter >50 mm compared with ≤40 mm. These findings support previous work from additional groups showing a correlation between LA size and the presence of AF in patients with HCM.16–18,29–31 LA volume has been associated with AF in a cohort of 427 patients with HCM (OR 1.062, 95 % CI [1.026–1.104]).32 Tani et al. demonstrated that a maximum LA volume of ≥56 ml identified patients with HCM and paroxysmal AF with a sensitivity of 80 % and specificity of 73 %.33 Furthermore, LA volume has been shown to identify those with HCM and normal pump function who are at risk of poor outcomes (LA volume/body surface area ≥40.4 ml/m2, sensitivity 73 % and specificity 88 %), including the risk of sudden cardiac death.34 LA enlargement is commonly seen in HCM and has been suggested to be a consequence of impaired diastolic function.35
McKenna et al. demonstrated right-sided involvement in 44 % of patients with HCM.36 However, these findings have not been confirmed, and the underlying mechanism and importance remains unclear. Despite this, Doesch et al. suggest this as an important prognostic factor for the development of AF in HCM.37 In a cohort of 98 patients with HCM (38 [39 %] with AF), cardiovascular magnetic resonance revealed reduced tricuspid annular plane systolic excursion and increased right atrial size were associated with the development of AF. However, this group did not directly quantify right ventricular hypertrophy.
Increasing age and worsening symptoms of congestive heart failure (NYHA class III or IV at diagnosis) have both been shown to be independently associated with the development of AF (OR 2.3, 95 % CI [1.4–3.7] and OR 2.8, 95 % CI [1.3–6.1], respectively).16 The prevalence of AF has been shown to increase with age in HCM cohorts; Losi et al. demonstrated an increase from 4.3 % in those <50 years of age to 13 % in those >60 years of age.18 Importantly, this group also highlights a large proportion of AF cases in an otherwise young population. An association between AF and increased age has similarly been reported in other large cohorts.25
Obstructive phenotypic presentation is variable in HCM.16,38 It has been demonstrated in several patient cohorts that LV outflow tract obstruction (LVOTO) is associated with increased risk of AF, in line with the expected physiological outcome associated with LVOTO. Indeed, LVOTO has been suggested to have a role in LA remodelling due to increased mitral regurgitation.39
It is well recognised that the range of mutations leading to the development of HCM can significantly alter the resultant phenotype.40 As such, it has been hypothesised that differential genetic mutations may explain some element of the heterogeneity witnessed in the development of AF within the HCM population. The Arg663His (rs371898076) mutation in the myosin heavy chain beta (MYH7) gene was shown to correlate with a high prevalence of AF (46 %) in a 24-patient cohort over a 7-year follow-up period.41 Mutations in the angiotensin-converting enzyme (ACE) gene have also been associated with the development of AF in patients with HCM.42 A summary of HCM features associated with AF development is detailed in Figure 1.
The Role of Atrial Fibrillation in Hypertrophic Cardiomyopathy Outcomes
Yang et al. demonstrated that AF was a risk factor for the development of cardiovascular events (a composite of sudden cardiac death, hospitalisation for heart failure, and stroke) on univariate analysis; however, on multivariate analysis, it was not found to be an independent predictor.29 In patients undergoing surgical relief of LVOTO, post-operative AF was associated with increased risk of a composite endpoint (death, appropriate ICD discharge, sudden cardiac death resuscitation, stroke and admission for congestive cardiac failure; hazard ratio [HR] 2.12, 95 % CI [1.37–3.34]).20 AF has also been found to be associated with worse survival in a cohort (N=1,069) of patients with HCM (HR 1.44, 95 % CI [1.20–1.71]).25
Analysis undertaken in a combined cohort from Italy and the USA demonstrated an increased risk of HCM-related death in patients with comorbid AF (OR 3.7, 95 % CI [1.7–8.1]).16 In a sub-group analysis, those who developed AF at ≤50 years of age had an increased risk of HCM-related mortality and progression of symptoms (1.7- and 1.5-fold, respectively). Increased HCM-related mortality rates17,43 and symptom progression related to the development of AF17 have also been reported by other groups. Indeed, stroke associated with AF was found to be the cause of 13 % of HCM-related deaths in a consecutive cohort of 744 patients with HCM.44
Treatment of Atrial Fibrillation in Hypertrophic Cardiomyopathy
Given the association between the development of AF and significant outcomes in HCM, prompt treatment of AF is required. In those with haemodynamic instability, electrical cardioversion is recommended,5 as with patients without HCM who develop AF.9 There is limited evidence to support specific treatment regimens for rate or rhythm control of AF in patients with HCM. Beta-blockers, diltiazem and verapamil are all recommended without significant evidence to support their efficacy in this patient group.5,9 However, given the likelihood that AF is highly symptomatic in HCM, conversion to sinus rhythm is considered beneficial. Amiodarone has been shown to be safe for use in patients with HCM,45 although long-term treatment is complicated by the sideeffect profile that is common with this medication. In addition, evidence for efficacy in this situation is derived primarily from non-randomised trials and is not overwelming.16,23,46 Disopyramide, recommended as a second-line therapy for symptomatic LVOTO,47 can be considered for the treatment of AF in patients with HCM;5 however, caution is needed in light of the potential for enhanced atrioventricular conduction and associated increased ventricular rate in AF.
The use of catheter ablation in patients with HCM to prevent AF recurrence has been shown to be potentially beneficial in a number of small studies.48–50 Success rates >60 % at 1 year have been reported. However, Di Donna et al. demonstrated that despite such overall success rates, redo procedures were required in 52 % of patients and antiarrhythmic medication was continued in 54 %.49 These results are not dissimilar to those seen in the general AF population. McCready et al. demonstrated that HCM was an independent risk factor for AF recurrence following multiple procedures (HR 2.42, 95 % CI [1.06–5.55]).51
Hypertrophic Cardiomyopathy and Stroke Risk
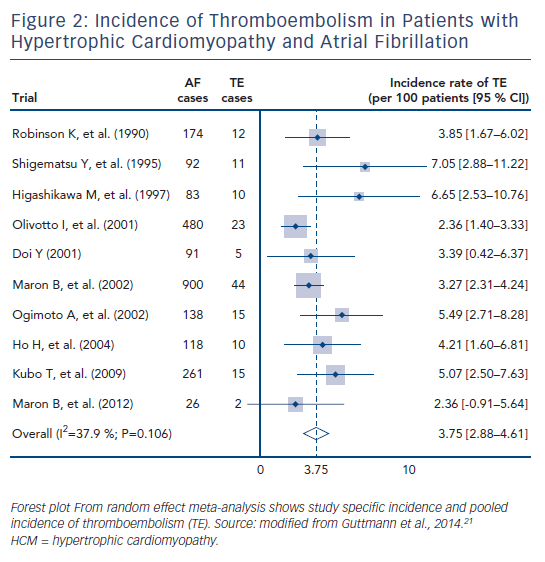
The risk of stroke in patients with HCM is well recognised, with Furlan et al. demonstrating a 7 % risk of cerebrovascular events over an average follow-up of 5.5 years.52 Incident rates of stroke in HCM, irrespective of AF diagnosis, have been estimated as 2.5 %/year.30 Compared with patients with HCM in sinus rhythm, those in AF were shown to have an eightfold increase in stroke risk (21 versus 2.6 %) in a 480-patient cohort (107 AF cases) over a follow-up period of 12.6 ± 7.7 years; thromboembolic events in patients with AF occurred on average 3.5 ± 3.4 years after AF diagnosis.16 This is supported by data from a Japanese cohort that demonstrated a 3.9-fold increased risk of stroke in patients with HCM and AF (23.0 versus 5.9 % at 5 years; p<0.01).53 High risk of stroke in the HCM population is further supported by additional groups.30,42,54–57 A meta-analysis of this topic area determined an overall annual incidence of stroke in patients with HCM and AF of 3.75 % (see Figure 2).21 However, despite the inclusion of 20 studies in this area, there were only 296 cases of thromboembolism from a pool of 6,102 HCM cases.
In a large retrospective cohort study (n=4,921), Guttmann et al. demonstrated that, having excluded those with prevalent AF, 2.2 % of patients with HCM developed thromboembolic events (cerebrovascular accident [CVA], transient ischaemia attack or peripheral emboli) within 5 years.58 In addition, in patients with AF, the presence of HCM is a strong independent risk factor for the presence of ischaemic stroke (52.6 versus 15.3 %; p<0.001).53 This increased risk is recognised in the Japanese Circulation Society’s HCM (2012) and AF (2013) guidelines, which recommend anticoagulation in all patients with HCM and AF.59,60
Stratification of Thromboembolic Risk in Hypertrophic Cardiomyopathy
Risk stratification for the incidence of stroke in AF has been a central component of guidelines issued by major cardiology societies over the past decade.9,60,61 In inividuals without HCM, this has included recognition that there is a population of individuals with AF who remain at low risk of stroke.9 All patients with HCM developing AF are considered to be at high risk of thromboembolic events. However, a consensus on what constitutes an increased risk of stroke in the HCM population has yet to be clarified. The current literature suggests several independent risk factors for the development of stroke in patients with HCM and AF (see Table 1).
LA diameter, as well as being associated with the development of AF itself, has also been shown to be a risk factor for thromboembolic outcomes.17,54,58 Notably, each 1 mm increase was shown to increase the risk of stroke-related death (HR 1.10, 95 % CI [1.00–1.20]).17 Increased LA size has also been suggested as an independent risk factor for thromboembolic events in patients with HCM without diagnosed AF.54
Increasing age is a recognised risk factor for stroke both within the general population, and particularly those with AF.9 Increasing age has been shown to be associated with increased risk of thromboembolic events in patients with HCM.30,54,58 However, it should also be noted that AF has been demonstrated at significantly younger ages in patients with HCM, and a significant number of thromboembolic events occur in this younger population.30 In support of this, Olivotto et al. reported that the risk of stroke was higher in patients ≤50 years of age.16
The presence of congestive heart failure symptoms is recognised as a risk factor for cerebrovascular events in the AF population. A similar position in the HCM population is supported by Maron et al. who demonstrated that the presence of NYHA class III–IV was independently associated with increased risk of stroke.30
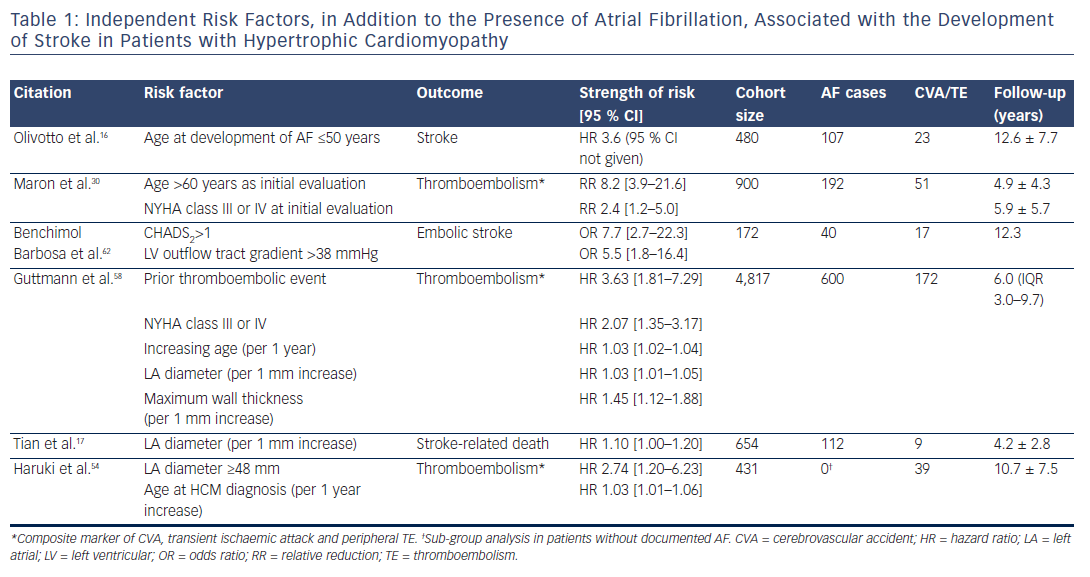
Using a list of pre-specified risk factors, Guttmann et al. were able to develop a risk model for predicting the development of thromboembolic events in patients with HCM.58 This model included age, presence of AF, previous thromboembolism, presence of congestive heart failure symptoms, vascular disease, LA diameter and maximal ventricular wall thickness. The authors described good correlation with the incidence of thromboembolic events. Although this model is a useful addition to the discussion of anticoagulation in this population, the complexity makes its use potentially cumbersome.
Some authors have previously advised using some elements of currently or previously established risk stratification tools in the general AF population. Benchimol Barbosa et al. found that a CHADS2 score >1 was associated with increased risk of CVA and have advocated its use as part of a score for the incidence of CVA in the HCM popuatlion.62 Inoue and colleagues, when assessing thromboembolic rates in those with non-valvular AF, have advocated a single point for the presence of HCM to the CHADS2 score;63 however, they failed to define a clear threshold at which point anticoagulation became necessary; instead assigned patients to low-, moderate- and high-risk categories. A low CHA2DS2-VASc score has been suggested as an appropriate marker for identifying little risk of thromboembolism in patients with HCM and AF. A CHA2DS2-VASc score ≤1 was associated with an annual thromboembolic incidence of 0.9 %;64 this is in line with thresholds of anticoagulation with non-vitamin K antagonist oral anticoagulants (NOACs).
However the use of traditional scores in risk stratifying stroke risk in HCM is not proposed in current guidance issued by the European Society of Cardiology (ESC) or Japanese Circulation Society (JCS).5,59 This position is supported by evidence showing a poor correlation between CHA2DS2-VASc score and the development of thromboembolism in a small sub-population of un-anticoagulated patients with HCM and AF (n=222).58 However, it should be noted that within this group there were only 21 events in total and no strong conclusions can be derived from this analysis.
Given the strong burden of evidence supporting a high risk of thromboembolism in patients with HCM who develop AF, such patients should be identified early. To date, no research has undertaken the prophylactic anticoagulation of patients with HCM and highrisk features for the development of AF. However, this may be an appropriate management strategy if such a population can be adequately defined.
Choice of Anticoagulant in Patients with Hypertrophic Cardiomyopathy
There is no randomised controlled trial assessing the role of anticoagulation among patients with HCM. Evidence is limited to that from small cohort studies, which show that the use of anticoagulation in patients with HCM and AF reduces the risk of thromboembolic events. Olivotto et al., in a cohort of 107 patients with HCM and AF, demonstrated a reduction of stroke from 39 % (n=11) in untreated patients to 10 % (n=6) in those treated with warfarin (p=0.001).16 This is in line with findings from a cohort of 200 patients with HCM and AF, where a reduction in the cumulative incidence of stroke was demonstrated with anticoagulation (31 % without anticoagulation [n=33] versus 18 % with warfarin [n=15]; p<0.05).30 Of note, patients on antiplatelet agents had no significant reduction in stroke risk, which is in line with findings in the general AF population.5,65 The role of anticoagulation is supported by other data showing a reduced risk of stroke when anticoagulated with warfarin (31–18 %).30
At present, no data are available from randomised controlled trials on the effectiveness of NOACs in reducing thromboembolic risk in this population. Among the four major prospective trials assessing the efficacy of NOACs versus warfarin in AF, patients with HCM were not included in the analyses.66–69 Large ‘real-world’ analyses of NOAC therapy have also failed to provide any specific discussion of patients with HCM.70,71 Noseworthy et al. examined a retrospective cohort of patients with HCM on anticoagulation and found no significant difference between NOACs and vitamin K antagonists in the rate of ischaemic stroke (HR 1.37, 95 % CI [0.40–4.67]) or major bleeding (HR 0.75, 95 % CI [0.36–1.57]).72 Furthermore, a recent post hoc subgroup analysis of the Randomised Evaluation of Long-term Anticoagulation Therapy (RE-LY) study has shown that the presence of LV hypertrophy determined by ECG criteria lead to decreased warfarin efficacy (dabigatran 150 mg versus warfarin HR 0.48, 95 % CI [0.29–0.78]).73 Although this analysis did not examine patients with HCM directly, the findings do suggest they may benefit from NOAC therapy.
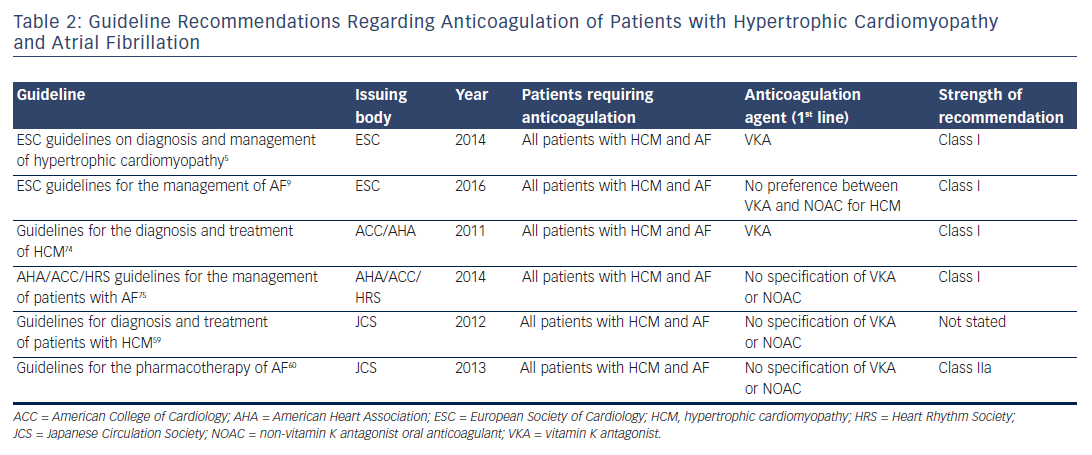
Given the strong evidence for their use in the AF population, NOACs have been recommended as second-line agents in patients with HCM and AF.5 However, this guidance remains unaligned between major guideline organisations. The American College of Cardiology (ACC), American Heart Association (AHA), ESC, and Heart Rhythm Society (HRS) uniformly recommend anticoagulation of all patients with HCM who develop AF (see Table 2). However, only in the most recent ESC guidelines discussing this patient group has the use of either vitamin K antagonists or NOAC anticoagulation been recommended.9
Conclusion
AF represents a common comorbid condition or complication in patients with HCM. As in the general population, AF is associated with significant morbidity from thromboembolic events and consequent mortality. The risk of thromboembolic events is higher than in the general population with AF and, although some independent risk factors have been identified, it is recommended that everyone with AF and HCM should be anticoagulated to mitigate this risk. However, the lack of data derived from randomised controlled trials or large-scale cohort studies emphasises the importance of and need for prospective registries with regards to the development of AF and its associated downstream outcomes. Given the burden of AF in the HCM population, and the high risk of associated thromboembolic stroke, it is now necessary to focus on identifying patients at high-risk of developing AF such that prophylactic anticoagulation can be considered.








