AF is associated with an increased risk of thromboembolic events.1,2 Risk scores are used to identify patients at high risk of such complications who may benefit from anticoagulation therapy.3 However, despite a broad range of anticoagulant options and improved uptake in anticoagulation over the past decade, there are limitations to this approach. First, there is a significant proportion of high-risk patients who have haematological disorders, frailty or previous major bleeding, whose high risk of bleeding precludes the use of anticoagulants.4 Second, there are patients who may continue to suffer thromboembolic events despite receiving appropriate guideline-directed anticoagulation therapy.5 Third, compliance and adherence with drug therapy may be suboptimal; discontinuation rates in randomised controlled trials (RCTs) of direct oral anticoagulants (DOACs) are between 21 and 27%.6–9 Therefore, there is the need for an alternative treatment strategy for these patients.
The majority (>90%) of thrombus formation in AF has been shown to originate from the left atrial appendage (LAA).10,11 As a result, surgical closure of the LAA has been performed in an attempt to reduce stroke risk.12 More recently, less invasive techniques, such as percutaneous LAA occlusion, have been developed. In this review, we aim to discuss the provision of LAA occlusion in the UK.
Demand for Left Atrial Appendage Occlusion in the UK
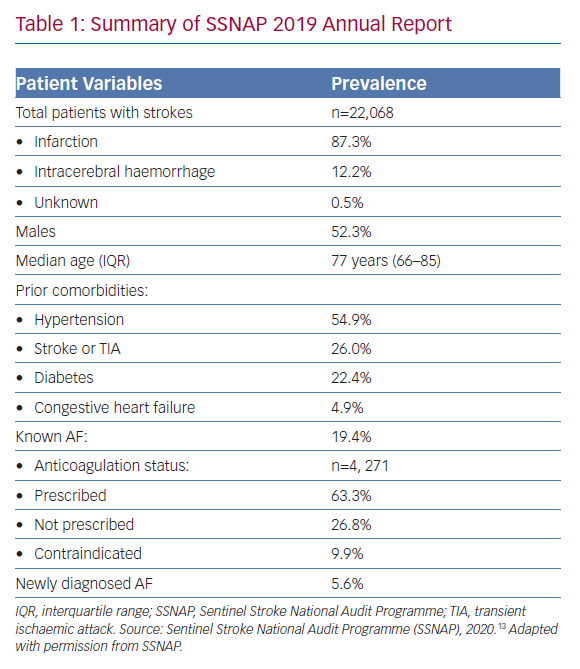
The Sentinel Stroke National Audit Programme (SSNAP) is a national quality improvement project that has recorded stroke data for most of the UK since 2012. In its most recent 2019 annual report (Table 1), it found that over a third of patients with known AF were not on oral anticoagulation therapy prior to their stroke presentation (n=1,566, 36.7%).13 Although this proportion has declined significantly compared with previous years – 61.6% in 2014 – it is still high enough to cause concern. Given that the majority of patients with AF and stroke were aged ≥70 years (68.9%) and suffered from hypertension (54.9%), it is unlikely that low perceived stroke risk was the main reason for the lack of anticoagulation among these patients. Although the exact reasons remain unclear, the report did highlight that many of these patients were not anticoagulated due to contraindications (n=421, 26.8%). This figure would chime with the observation from primary care’s Quality Outcomes Framework data that about 6% of the overall AF cohort in the UK are deemed to have contraindications to anticoagulation, although this was lower than the estimated 12% from the US.14,15 Furthermore, the SSNAP data showed that 2,705 strokes occurred in AF patients who were already receiving anticoagulation therapy (12.3% of the total cohort).
A UK population-based cohort study of 11,481 patients with AF who were treated with a DOAC between January 2012 and December 2016 found that almost a third of patients had discontinued DOAC treatment within 1 year.16 The majority of these patients (60.4%) had a gap of at least 30 days without stroke protection before eventually reinitiating treatment with a vitamin K antagonist or DOAC. However, a significant percentage (n=813, 7.1%) still remained without anticoagulation following this period. Similar discontinuation rates were reported in other non-UK cohorts.17,18 Overall, these data demonstrate that there is a clear demand for LAA occlusion therapy among patients in whom anticoagulation is either not tolerated or is contraindicated, with a further potential role in those with anticoagulation-resistant strokes.
Service Delivery in the UK
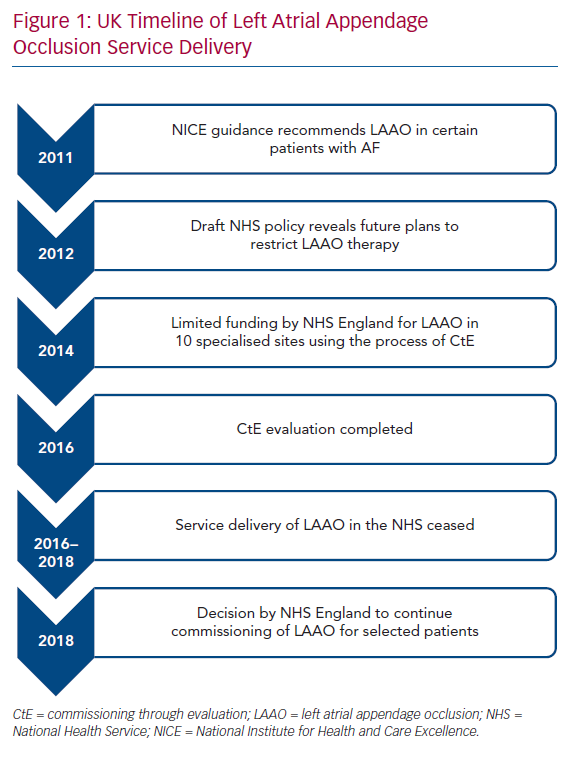
The UK population is covered by the National Health Service (NHS), a publicly funded healthcare system. The National Institute for Health and Care Excellence (NICE) is responsible for appraising evidence and providing guidelines for clinicians in England and Wales. In 2011, NICE determined that there was inadequate evidence to support percutaneous LAA occlusion as an adjunct for stroke prevention in AF.19 However, it recognised that certain patients may be unable to tolerate anticoagulation and permitted the use of LAA occlusion in those circumstances.
One year later, a draft policy in the NHS revealed that there were plans to put restrictions on the routine commissioning of LAA occlusion.20 Concerns were raised from healthcare professionals and health charities about this and NHS England decided upon a multicentre observational registry using the process of Commissioning through Evaluation (CtE).21 The purpose of the single-arm CtE registry was to evaluate LAA occlusion as a possible treatment option for patients with AF at high risk of stroke who have contraindications to anticoagulation therapy. As a quid pro quo for contributing to the registry, 10 specialised centres in England with cardiac surgery facilities were granted limited funding to perform LAA occlusion between October 2014 and September 2016. After this period, there was an 18-month interval during which LAA occlusion in the NHS essentially ceased due to lack of funding while data from the CtE registry were analysed and reviewed by the specialised commissioning group. In June 2018, a decision was made by NHS England to support commissioning of LAA occlusion in selected patients with non-valvular AF and high thromboembolic risk, defined as having a CHA2DS2-VASc score of ≥2, where there is a physician-assessed contraindication to oral anticoagulants.22 This included patients with anticoagulation-resistant strokes. Under this policy, patients who had a Rockwood frailty score of ≥6 or a life expectancy of less than 3 years were deemed unsuitable for LAA occlusion. All procedures undertaken were to be recorded on a national registry to allow prospective evaluation of long-term outcomes. The plan was to perform 400 cases in the first year at the same 10 centres that were part of the CtE process, increasing to 1,200 a year over 5 years with the approval of additional centres following another round of selection in summer 2019. At the time of writing this review (February 2020), no more centres have been commissioned. Recently, the Scottish Health Technologies Group released a report advising that NHS Scotland should offer LAA occlusion to similar patients.23 Figure 1 shows a timeline of the LAA occlusion service delivery in the UK.
Access to Left Atrial Appendage Occlusion
Broadly speaking, the eligibility and funding criteria for LAA occlusion in the UK resemble that of France, the US, Australia, Poland and Canada.24–26 The fundamental difference, however, is in the restriction in the number of centres commissioned to provide LAA occlusion in the UK set at 10 as this puts a significant constraint on the provision of this service at a population level. In Germany, provision of LAA occlusion is dependent on individual insurance providers and is not subject to restrictions. In New Zealand, there are severe restrictions imposed on LAA occlusion in the public sector, but patients with anticoagulation-resistant strokes and a high risk of bleeding may be covered by private health insurance.27
Data on Left Atrial Appendage Occlusion
The final report of the CtE registry was produced in early 2019 and included 525 patients with AF who underwent LAA occlusion.21 Virtually all cases were performed under general anaesthetic (99.4%) and with intraoperative transoesophageal echocardiographic imaging (99.5%). Median fluoroscopy time and procedural duration in minutes were 10 (inter quartile range [IQR] 7–15) and 75 (IQR 57–110), respectively. Overall procedural success was 89% with a periprocedural mortality risk of 1%. Median length of stay was one night with 22.4% of patients requiring an extended admission (≥2 days). No differences in outcomes were seen between the various devices used. Risk of ischaemic stroke during follow-up was significantly reduced compared with that predicted from validated risk scoring systems, affirming the role of LAA occlusion in patients with AF who have contraindications to anticoagulation therapy. Furthermore, subsequent linkage of 460 patients with two UK datasets (Hospital Episode Statistics and Office of National Statistics) produced comparable data with the registry, adding confidence to the results.21
Based on our experience at a large tertiary centre in the UK, LAA occlusion can be performed with a high procedural success rate (82/83, 98.8%) in patients with contraindications to anticoagulation therapy.28 The procedure appeared to result in a reduction of stroke rates compared with historical cohorts with a corresponding risk profile. In those who did have a stroke despite LAA occlusion, none were disabling, and all patients made a full recovery. This finding supports the notion that LAA occlusion may be associated with fewer AF-related strokes, as well as lesser severity of strokes when they do occur.29–32 It was our practice that all procedures were performed jointly by a consultant electrophysiologist and interventional cardiologist under transoesophageal echocardiogram and fluoroscopic guidance. Post-procedural dual antiplatelet therapy was mandated for 6 weeks, followed thereafter by single antiplatelet therapy up to 6 months. Most patients in our centre were kept in for overnight monitoring and discharged the following day, with a mean length of hospital stay of one day. More recently, Williams et al. demonstrated that LAA occlusion can be performed safely as a day-case procedure with very low rates of complications and readmissions.33
A retrospective registry by Betts et al. reported on outcomes from 371 patients with AF who underwent percutaneous LAA occlusion at eight centres in the UK prior to the period when the CtE registry was active.34 The follow-up period was over 24 months. Overall procedural success was 92.5% with an annual relative risk reduction based on predicted risk profiles for ischaemic stroke, thromboembolic events and major bleeding of 90.1%, 87.2% and 92.9%, respectively. The number of LAA occlusions undertaken at each centre varied significantly with a median of 40 cases (IQR 5–145). This suggests that some centres in the UK performed very few procedures during the study period, a factor which has been shown to be associated with worse outcomes.35
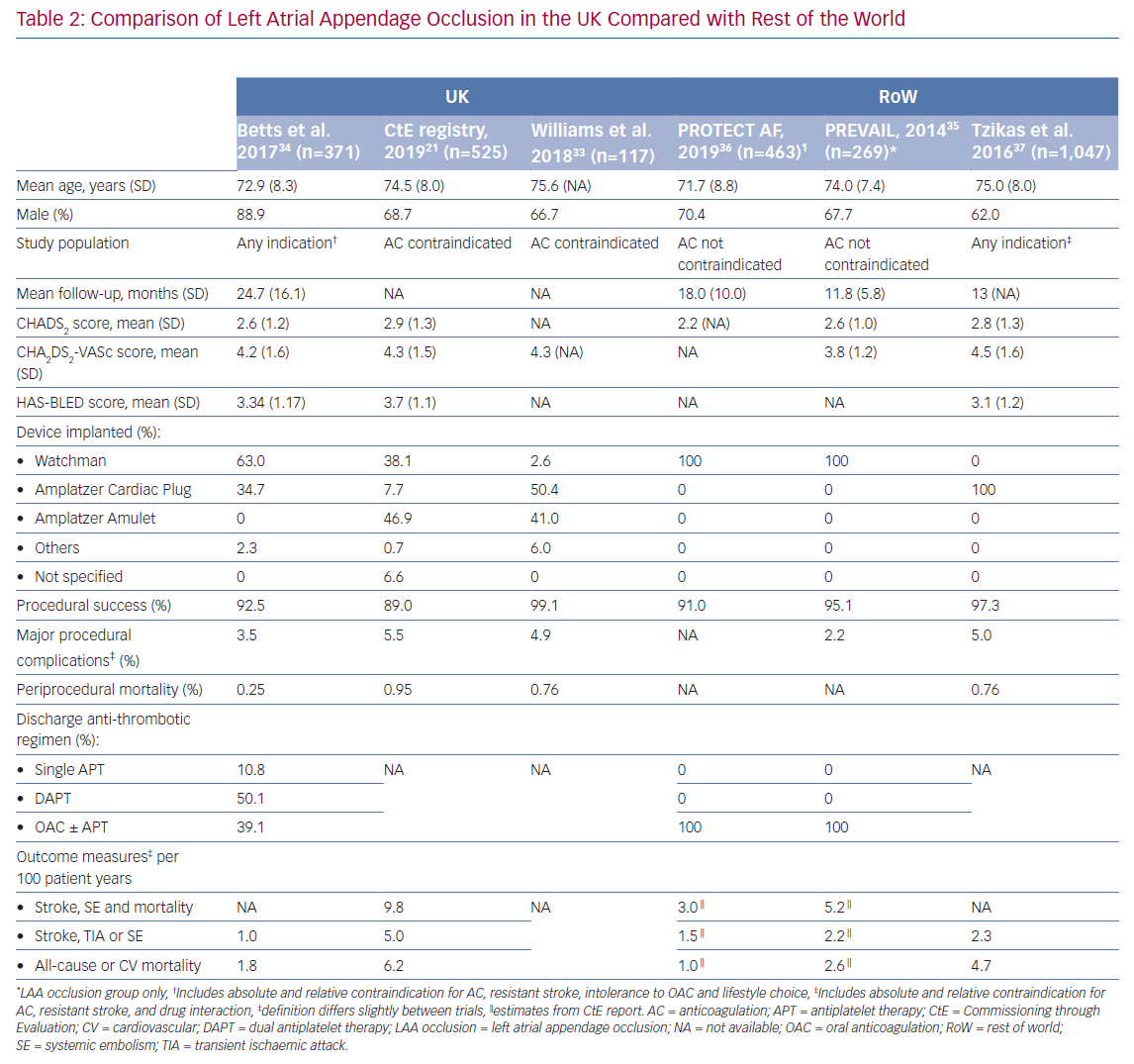
The UK-specific data appear broadly in agreement with that from international registries (Table 2).21,33–37 The relatively high periprocedural mortality rates reported in the CtE registry and study by Williams et al. was also observed by Tzikas et al. and may be related to an initial learning curve with the procedure.21,33,37 Overall, a direct comparison of complication rates across studies may be inaccurate due to confounders related to differences in the inclusion criteria and baseline risk factors. With this in mind, the periprocedural mortality rates found in the aforementioned studies were greater than in the Registry on WATCHMAN Outcomes in Real-Life Utilization (EWOLUTION) study (NCT01972282).38 Worse primary outcomes observed in the real-life registries compared with randomised trials may be explained by recruitment of patients with a higher risk of stroke, along with greater prevalence of comorbidities causing contraindication to oral anticoagulation.35,36
Despite evidence to support the role of LAA occlusion in patients with AF who have contraindications to anticoagulation therapy, there are several factors to be considered. About half of the cases in the UK were performed using the Amplatzer Amulet device (Abbott). However, results from RCTs are currently only available for the Watchman device (Boston Scientific).35,36 Furthermore, these trials excluded patients who were considered unsuitable for anticoagulation, thereby further limiting generalisability of their results to patients receiving LAA occlusion.
In general, the use of an epicardial approach for LAA occlusion remains poorly explored. Nonetheless, this offers an interesting prospect as the relatively high periprocedural complication rates may potentially be balanced by the absence of an intracardiac device, thereby negating the need for even short-term anticoagulation and the risk of device-related thrombus.39–41 Currently, the majority of the data on LAA occlusion are derived from real-world registries that may be subject to selection and reporting bias. There are limited studies directly comparing LAA occlusion to placebo and additional well-designed RCTs are needed. There are two ongoing RCTs that may provide some insight on the matter – Prevention of Stroke by Left Atrial Appendage Closure in Atrial Fibrillation Patients After Intracerebral Hemorrhage (STROKECLOSE; NCT02830152) and Assessment of the WATCHMAN™ Device in Patients Unsuitable for Oral Anticoagulation (ASAP-TOO; NCT02928497) but results are not expected for several years. It has also been suggested that LAA occlusion may be feasible in patients with proven LAA thrombus although this needs further evaluation.42
Cost Efficacy of Left Atrial Appendage Occlusion
In a publicly funded healthcare system, such as the NHS, the cost-effectiveness of LAA occlusion is an important consideration. Using recent estimates, the cost of each procedure was about £11,600.43 This represented an increase of 78% compared with the lifetime cost of medical therapy with antiplatelets alone. However, when the higher initial cost of the procedure is balanced against a reduction in medical and social care expenditure from lower stroke rates, it is forecasted to be cost neutral over a 15-year period. When compared with the cost of medical therapy with anticoagulants, LAA occlusion was found to achieve cost parity between 4.9 years versus dabigatran and 8.4 years versus warfarin.44 The study by Panikker et al. estimated that LAA occlusion may save up to £7,194 at 10 years compared with other therapies. As such, the predicted remaining lifespan of individuals is an important factor when assessing their suitability for LAA occlusion. Similar cost benefits have also been demonstrated in studies in the US.45–47
In the current UK setting – and many other parts of the world – the majority of patients with AF are seen in primary care. This includes many patients who may be deemed unsuitable for anticoagulation by GPs. However, given the new policy changes and the unavailability of LAA occlusion until recently, many clinicians may not be aware that there exists an alternative for such patients. Estimates from NHS England predict that referral networks may require more than 5 years to become established and eventually only 10% of LAA occlusion-eligible patients will be considered for this treatment.22
Conclusion
Percutaneous LAA occlusion appears to be a viable option in patients with AF who have contraindications to anticoagulation therapy, which comprise 5-6% of the total AF population. Availability of this therapy is at present significantly restricted in the UK compared with many countries in western Europe and the US.
Clinical Perspective
- Percutaneous LAA occlusion is associated with a significant reduction in thromboembolic risk among patients with AF who have contraindications to anticoagulation therapy.
- Patients with AF who have high thromboembolic risk and are unable to tolerate anticoagulation, including those with anticoagulation-resistant strokes should be referred to a specialist for consideration of percutaneous LAA occlusion.
- The procedure is associated with a high initial cost that appears to be subsequently balanced against a reduction in medical and social care expenditure from lower stroke rates over a 10–15-year period.