Direct pacing of the atrioventricular conduction axis, usually known as the His–Purkinje system, is currently considered the most physiological of the methods available for restoring intrinsic atrioventricular conduction. Pacing the His bundle, the left bundle branch or the region surrounding it can restore physiological activation of the ventricles, thus restoring electrical synchrony caused by bundle branch block. Such pacing can also improve clinical outcomes in patients with heart failure.1–8 Pacing of this type corrects the situation either by delivering an impulse beyond the site of block or by penetrating beyond the area of block, and, hence, directly recruiting the fascicles of the left bundle branch. There is an emerging role for such pacing to achieve cardiac resynchronisation in addition to conventional biventricular pacing, particularly in patients with more proximal diseases of the conduction system.3–8 Recognition of these possibilities has underscored the current resurgence of interest in the topic of the anatomical relationships of the atrioventricular conduction axis, and their implications for pacing.9–15
Of course, the His bundle is but a part of the overall axis of specialised conduction tissue that, in the normal heart, provides the solitary myocardial connection between the atrial and ventricular myocardial compartments.16 The concept of corrective pacing is based on the notion that the abnormalities in conduction are due to proximal focal lesions producing either intra- or infra-Hisian lesions. The consequence of such lesions is complete atrioventricular dissociation, severe slowing of conduction or asynchronous conduction along pre-destined, longitudinally dissociated pathways, as elegantly demonstrated by Narula in 1977.17
Successful pacing with early capture of the conduction system or biventricular pacing results in simultaneous physiological activation of both ventricles. This prevents the untoward effects of non-physiological chronic right ventricular apical pacing.11 The procedure, however, has some limitations. These include the narrow target zone of the conduction axis, which varies greatly between individual patients, along with the dense nature of the fibrous tissue that surrounds the penetrating bundle. These anatomical features explain, in part, the technical challenges of the procedure, along with the frequent occurrence of high and unstable pacing thresholds subsequent to implantation.4–6 They also account for the delayed rise in thresholds, abnormal sensing and permanent acute or chronic damage to the atrioventricular conduction axis itself. Taken together, as demonstrated during mid-term follow-up, they produce an unacceptable need for repositioning of the leads in one-tenth of patients.18
Because of these inherent limitations associated with attempts at pacing the His bundle, which is either the penetrating or non-branching components of the axis, direct pacing of the left bundle branch has recently emerged as an alternative option.1,5 Such pacing, by means of intraseptal lead fixation deep into the left side of the muscular ventricular septum, can bypass distal pathological lesions involving the left bundle branch and its surrounding regions.7 The procedure is aimed at direct capture of the fascicles of the left bundle branch, thus preserving or restoring physiological activation of the left ventricle. The narrow QRS duration achieved is likely due to the dense arborisation of the left bundle branch, which permits rapid conduction and simultaneous capture of the greater part of the left ventricle.11 The procedure is associated with technically easier implantation of the lead, a shorter learning curve and higher rates of success of implantation. It produces stable and reliable lead parameters.
It is axiomatic that precise knowledge of the anatomy and histology of the atrioventricular conduction axis is critical for those seeking to achieve such direct pacing. With this in mind, we have reviewed our recent investigations of the anatomical variations involving the axis, features necessary for a proper understanding of pacing of the conduction system in the human heart.
The Area of the His Bundle: Anatomical Considerations
In 1893, Wilhelm His Jr was the first to report the location of the solitary strand of specialised myocardium providing electrical connection between the atria and ventricles, but he was unaware of its atrial and ventricular extensions.11,19 It was not until 1906 that Tawara clarified the situation.16 He showed that the transition from atrial to the ventricular components occurred as the conduction axis entered the insulating tissues of the atrioventricular junctions. He described, for the first time, the extent of the solitary pathway of specialised myocardium, demonstrating the histologically distinct cells that conducted impulses from the atrial chambers to the apical ventricular myocardium.11,16
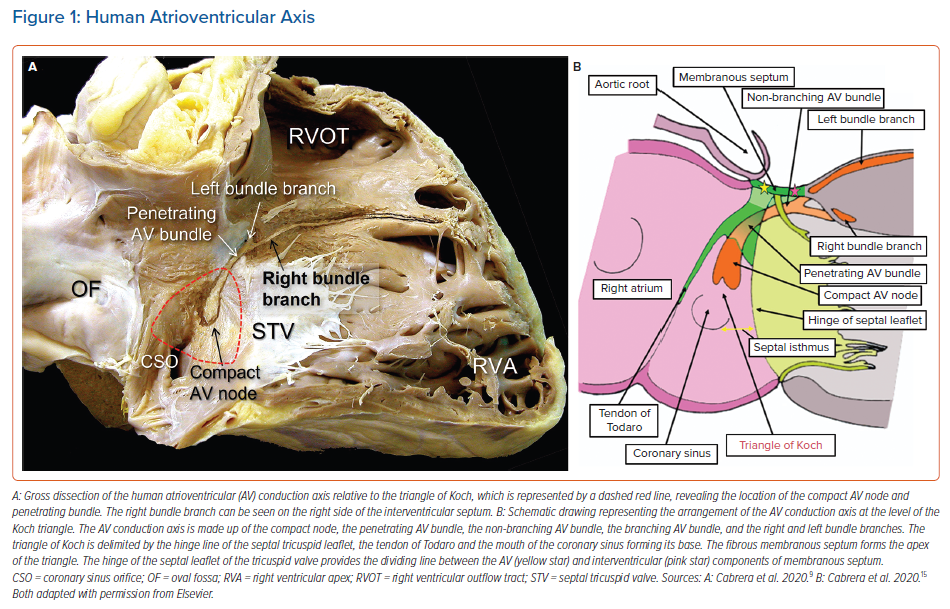
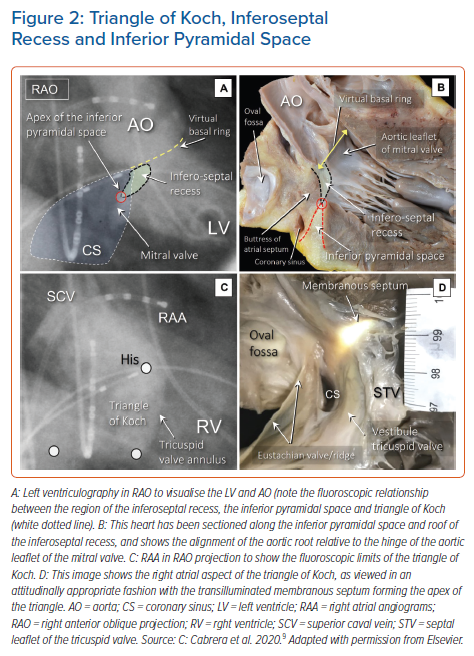
It is now well recognised that the atrial components of the conduction axis are located within the atrial wall of the triangle depicted by Koch. The area is part of the septal vestibule of the tricuspid valve. It forms the atrial wall of the inferior pyramidal space. It contains not only the atrioventricular node, but also its rightward inferior extension and overlies the septal inputs. The paraseptal area is an overlap of the atrial and ventricular musculatures, with a superior extension of the inferior atrioventricular groove separating its muscular walls. The triangle is limited cranially by the fibrous part of the septal components, usually known as the membranous septum. The mouth of the coronary sinus, and the septal isthmus, form the base of the triangle (Figures 1 and 2).20–25 The atrial wall of the triangle is separated from the underlying crest of the muscular ventricular septum by the fibro-adipose tissue that fills the inferior pyramidal space.
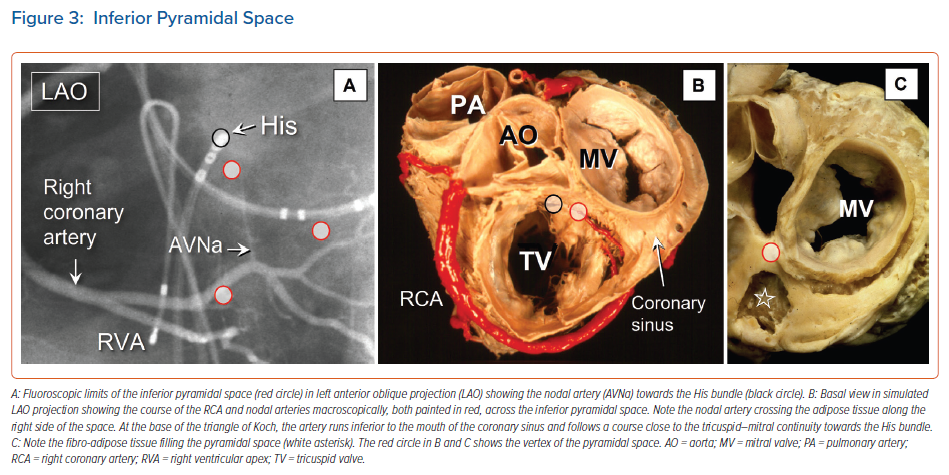
When considered in attitudinally appropriate fashion, the pyramidal space is the superior continuation of the inferior atrioventricular groove (Figures 2 and 3). It is bounded on the atrial side by the diverging vestibules of the mitral and tricuspid valves. Its ventricular boundary is formed by the crest of the muscular ventricular septum. At its apex is found the inferior margin of the membranous septum, which is typically an atrioventricular partition as this level. It is often overlaid by a layer of atrial vestibular myocardium, which then separates the apex of the pyramidal space from an inferoseptal recess within the left ventricle (Figure 2). The tissues occupying the pyramidal space are themselves thickened at its apex, forming a fibrous insulating plate on the crest of the ventricular septum. The atrioventricular node becomes the penetrating bundle by passing through the fibrous tissues of the atrioventricular junctions, specifically through an area of fibrous continuity between the leaflets of the mitral and tricuspid valves (Figure 4).
In the majority of individuals, the apex of the pyramidal space is itself adjacent to the inferoseptal recess of the left ventricle. This latter space interposes between the aortic leaflet of the mitral valve and the septal surface of the left ventricle. It is roofed by an area of mitral-to-tricuspid valvar continuity that supports the base of the atrial septum. The conduction axis usually penetrates through this fibrous tissue, which then forms the rightward wall of the recess. The variation in the depth of the inferoseptal recess is itself reflected in the extent of observed off-setting of the leaflets of the mitral and tricuspid valves (Figure 2). This feature, in turn, influences the location of the node, and its transition to the His bundle within the apex of the triangle of Koch.9,21
It is the combination of knowledge of these anatomical features, and the site of recording of the His bundle electrograms, that will determine the optimal positioning of the pacing lead.
Anatomy Relevant to Direct Pacing of the Penetrating or Non-branching Atrioventricular Bundle
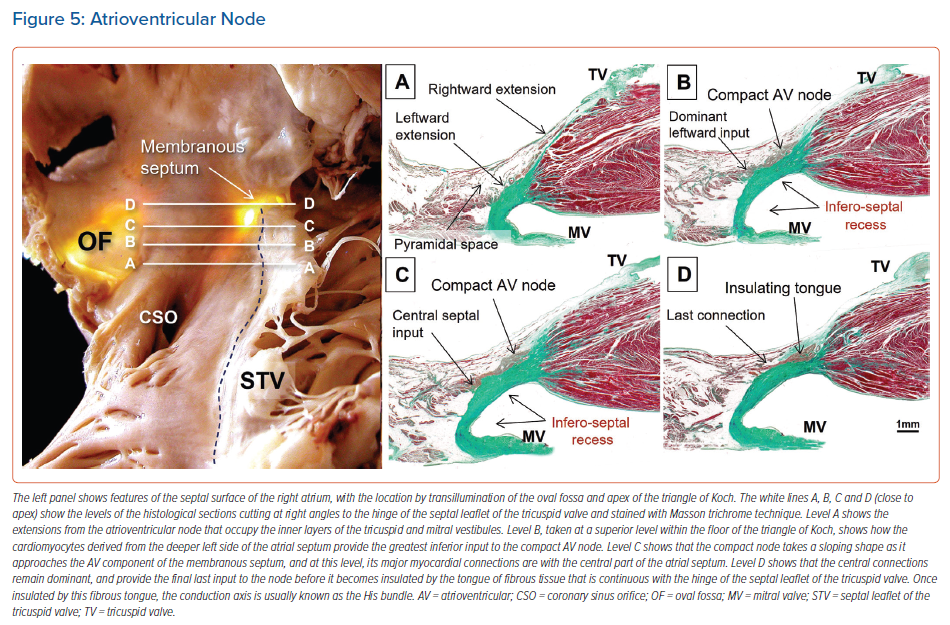
When searching for the optimal site for pacing of the penetrating component of the conduction axis, electrophysiologists should map both the atrial and ventricular aspects of the tricuspid valvar vestibule. In our anatomical studies, we have encountered marked inter-individual differences, with significant variations in the site of transition from the atrioventricular node to the non-branching component of the axis relative to both the hinge of the septal leaflet of the tricuspid valve and the atrioventricular septal structures. As we have emphasised, we followed the precedent of Tawara when taking the site of penetration into the insulating tissues provided by the continuity between the leaflets of the mitral and tricuspid valves as representing the transition from the node to the penetrating component of the axis (Figure 5).9,16 This penetration into the insulating fibrous tissues occurred superiorly in almost three-fifths of specimens, with the transition found inferiorly within the triangle of Koch in the remaining two-fifths of hearts.
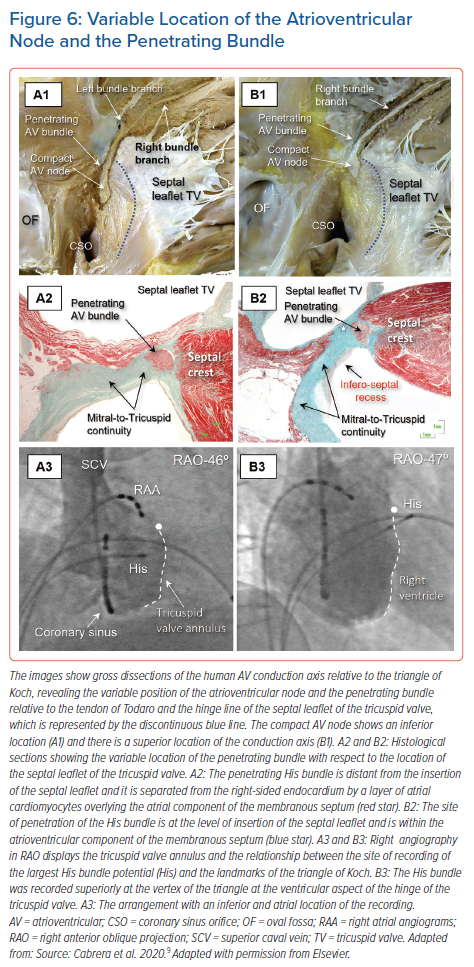
Consequently, we observed that the axis penetrated on the atrial side of the hinge-line in just over half of the hearts, whereas in the remainder, the transition was either at the hinge-line or on its ventricular aspect. This is in keeping with our findings regarding the location of the largest His bundle deflection relative to the septal leaflet and the angiographic vertex of the triangle of Koch (Figure 6).9,26 This is significant for those seeking to find the optimal site for direct pacing, since the conduction axis is generally mapped along the tricuspid vestibule in the triangle of Koch. It is intuitive to suggest that the point of transition will prove to be the optimal site for direct engagement of the so-called His bundle.
When assessed relative to the right-sided chambers, the site of penetration was found in an atrial location within the triangle in just over half of hearts, whereas penetration coincided with the hinge point of the septal leaflet of the tricuspid valve, adjacent to its zone of apposition with the anterosuperior leaflet in one-third of hearts. In the remaining one-sixth of hearts, the site of penetration was within the ventricular component of the membranous septum. In over nine-tenths of hearts having an inferior site of penetration, the axis penetrated at the atrial side, but was centrally located within the triangle. In contrast, in those hearts with a superior site of penetration, penetration occurred within the atrial tissues in only one-third of the cases. It was at the level of the hinge of the septal leaflet, or in its ventricular aspect, in the remaining two-thirds (Figure 6).9
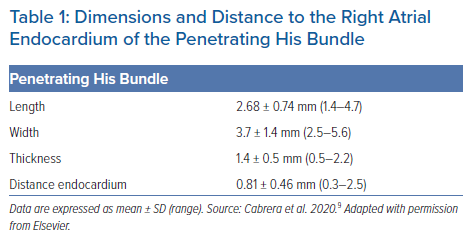
The adjacency of the conduction axis to the right-sided endocardial surface, and the atrial myocardial overlay, is itself a further important consideration for direct pacing of the conduction axis. Typical values for the length, width and thickness of the penetrating His bundle, and its distance from the right atrial endocardium, are shown in Table 1.9 Selective pacing results in exclusive capture of the non-branching component of the conduction axis, without myocardial capture. Non-selective pacing occurs when the adjacent atrioventricular and ventricular septal myocardium is captured alongside capture of the axis itself. When the conduction axis is not captured, but the pacing stimulus produces ventricular activation, then only the local myocardium is captured.7
The covering between the axis and endocardium provided by a layer of overlying right atrial myocardium was greater in hearts with an inferior transition from the node to penetrating bundle than in those hearts with a superiorly located transition. The latter arrangement was associated with a thicker surrounding connective component and less overlying atrial vestibular myocardium (Figure 6).
This latter point is obviously of significance in terms of sensing and threshold for those hoping to achieve direct pacing of the conduction axis. When the distal sensing electrode of the lead sits on the atrial side, it could oversense the atrial potential, with concomitant undersensing of the ventricular signal. As a consequence, the position of the lead may have an important impact on pacing parameters. Capture is known more often to be selective when the pacing lead tip is placed on the atrial side of the site of insulation. Positioning it as distally as possible can significantly help increase the sensing values.
Investigators had recently explored the relationship between the location of the tip of the pacing lead and the hinge of the septal leaflet of the tricuspid valve using cross-sectional echocardiography. Tang et al., for example, showed that the site of implantation of the leads has a significant effect on sensing values.27 They found that, on average, the distance from the tip of the leads targeting the axis to the attachment of the septal leaflet of the tricuspid valve was 3.16 ± 1.54 mm with an atrial location, but was 6.12 ± 2.21 when the lead was located at the ventricular aspect of the hinge. A ventricular location of the lead was linked to higher sensing values, but the achievement of selective pacing was greater when pacing was performed at the atrial side. In contrast, when the lead is deployed in the ventricular position, there is a greater chance of non-selective pacing, capturing both the conduction axis and the surrounding right ventricular myocardium. This latter result might prove to be safer. Other have suggested, however, that pacing on the atrial side can reduce any deleterious impact on the tricuspid valve, which is considered an advantage of direct compared with conventional pacing.13
The process of implantation for direct pacing of the conduction axis can often be challenging. In clinical practice, it is technically difficult to determine whether the pacing lead has crossed the hinge of the tricuspid valve during the implantation procedure. This is because standard fluoroscopy cannot determine the precise location of the site of penetration of the conduction axis.26,28,29 The standard procedure for implantation involves seeking a His bundle potential when using fluoroscopy in the right anterior oblique view. There is no clear anatomical marker for the site of penetration of the conduction axis when using this approach. The view does provide anterior and posterior orientations, and helps to localise the plane of the tricuspid valve. It is the view obtained in the left anterior oblique orientation, however, that identifies the septal contact of the catheter. In the right anterior oblique view, the translucent area that indicates the atrioventricular groove can be useful when seeking to approximate the location of the coronary sinus to the hinge of the leaflets of the tricuspid valve.12
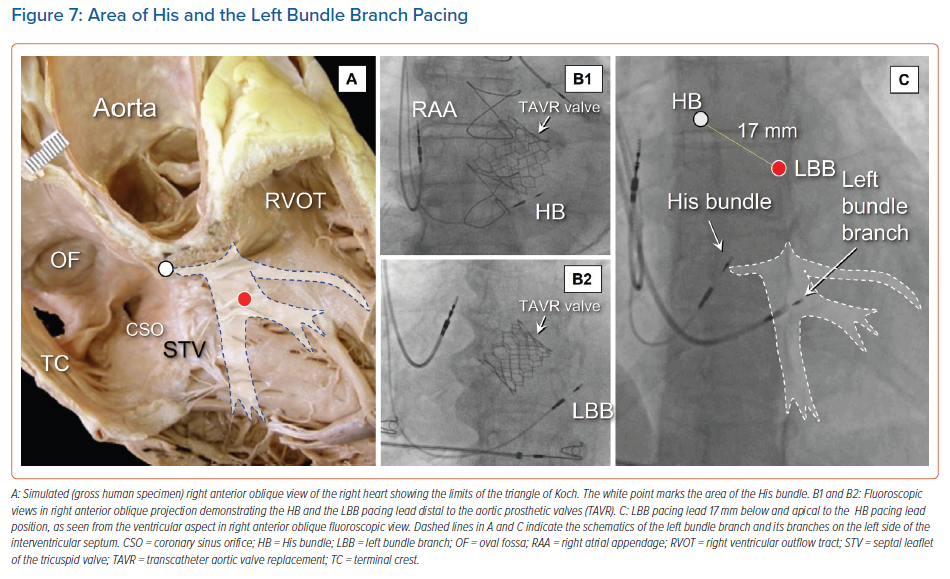
In some instances, the location of a prosthetic aortic valve can be used as an anatomical landmark for localising the conduction axis, thus improving implantation on the ventricular side (Figure 7).30 In particular, for individuals in whom self-expandable valves have been inserted by catheter, it seems reasonable when seeking to pace the fascicles of the left bundle branch to avoid placing the pacing lead in contact with the struts of the prosthesis. This should ensure no early damage to the lead, and avoid sensing issues due to noise detection. Clinical evidence to support this recommendation, however, is currently lacking. We have previously shown that right atrial angiography may help to identify the variable sites of recording of the conduction axis in relation to the limits of the triangle of Koch and the septal hinge of the tricuspid valve.26,29 The atrial and ventricular location of the His catheter in relation to the hinge of the valvar leaflets can readily be identified by this technique. Other authors have used injection of contrast into the right ventricle to provide for good delineation of all these structures.31 This approach may be of great help. It identifies the potential atrial as opposed ventricular sites of the conduction axis. Those using this approach found that capture was more often selective when the pacing lead tip was placed on the atrial side of the site of penetration. Such visualisation shortens the procedural and fluoroscopic times for implantation. Others have used tomographic reconstruction in a subgroup of patients undergoing successful His bundle pacing. The location of the tip of the lead was, in almost four-fifths of cases, on the ventricular aspect. An atrial position was most commonly linked to selective capture, although this was linked to increased atrial oversensing.32 Knowledge of the anatomical location of the site of penetration, therefore, can help guide optimal placement of the lead. It is linked to different physiological pacing characteristics (Supplementary Material Video 1).27,31
The Ventricular Components of the Conduction Axis
The conduction axis, having penetrated by passing through the fibrous tongue, producing continuity between the hinges of the leaflets of the mitral and tricuspid valves, continues as the non-branching component. In over three-quarters of individuals, this part runs for less than 3 mm along the crest of the muscular ventricular septum before giving rise to the fascicles of the left bundle branch. This part of the axis is embedded within the fibrous tissues forming the right wall of the inferoseptal recess. Variations in the location of this non-branching component relative to the crest of the muscular ventricular septum were assessed in detail by Kawashima and Sasaki.33 They identified three distinct patterns. In just over half of their specimens, the bundle ran along the lower border of the membranous part of the ventricular septum, usually covered with a thin layer of ventricular myocardium. In one-third, the bundle was separated from the membranous septum, being further covered by a thick myocardial layer. In the remaining one-fifth, the bundle was directly subendocardial, lacking any myocardial covering.
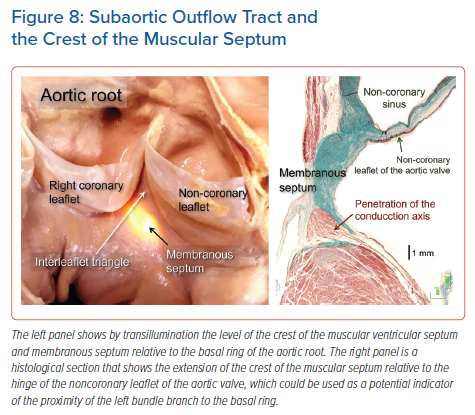
The arrangement of the fibrous membranous septum itself is also known to be variable.19,22,24,25 In many instances, the atrioventricular component, as we have discussed, is overlaid by a layer of atrial myocardium, which often includes the atrioventricular node itself. The conduction axis itself, having penetrated to enter the subaortic outflow tract beneath the circumference of the aortic root, usually gives off the right bundle branch at the level of the interleaflet fibrous triangle between the non-coronary and right coronary aortic sinuses. The right bundle branch is a thin cord-like structure. It typically takes a short course embedded within the muscular ventricular septum before emerging in the subendocardium of the right ventricle at the base of the medial papillary muscle, also known as the muscle of Lancisi.11 Some may identify this muscle as being ‘septal’, but this is potentially confusing, since multiple small muscles tether the septal leaflet of the tricuspid valve. The right bundle then courses towards the moderator band and anterolateral papillary muscle, arborising to supply the right ventricular myocardium.
The inter-individual variations in the location of the components of the conduction axis are of particular importance not only for identification of the site for optimal pacing of the penetrating bundle, but also for recognition of the origin of the ramifications of the left bundle branch. It is for this reason that identification of the site of penetration is itself of importance, even if direct pacing of the left bundle branch is the objective of the pacing procedure (Figure 7).
Pacing the Left Bundle Branch and its Surrounding Regions
Direct implantation of the pacing lead in one of the fascicles of the left bundle branch has recently emerged as a further strategy for patients with infra-Hisian block.1,2,5 Unlike direct pacing of the penetrating bundle, where the target zone for effective pacing is very small, the fascicles of the left bundle, despite presenting large inter-individual variations, are widely distributed. Indeed, the presence of the branching as an extensive subendocardial network may facilitate the implantation of a lead via the intraseptal approach.
In 2017, Huang et al. reported direct pacing of the left bundle branch.1 This was achieved by transseptal implantation of a lead, usually resulting in capture with low pacing thresholds. Using the location of the His recording as a reference, the initial site for implantation was up to 15 mm below the hinge of the leaflets of the tricuspid valve, as seen from the ventricular aspect, and apical to the position of the His potential in the 30° right anterior oblique fluoroscopic view. This emphasises that knowledge of the location of the site of penetration of the axis is also of critical importance in seeking to identify the optimal position to deploy the pacing lead.
Using this technique, it is necessary to penetrate deep into the muscular ventricular septum, so as to implant the lead on either the trunk or the ramifications of the left bundle branch. Such pacing also demonstrates selective, as opposed to non-selective, capture of the left bundle, or left ventricular septal pacing without direct capture of the conduction system.7 It may be very difficult, nonetheless, to differentiate the exact electrophysiological location of the pacing lead and the tissue captured. Technically speaking, furthermore, deep intramural pacing is not identical to direct capture of a fascicle of the left branch. Although little evidence exists due to obvious limitations of the lead deployment, we speculate that fascicular capture will be indicated when the initial deflection of the paced QRS is narrower than achieved using deep intramural pacing. Such differentiation, although theoretically interesting, may not always be possible in the clinical situation.
It is the rule for the component of the axis giving rise to the left bundle branch to be sandwiched between the inferior border of the membranous septum, and the crest of the muscular ventricular septum. Indeed, in many instances, the axis itself, as it gives off the fascicles of the left bundle branch, is embedded within the base of the membranous septum (Figure 8). The origin of the most cranial fascicle of the left branch typically marks the end of the branching portion of the axis. Not always, however, is the axis carried on the crest of the muscular septum. It was quite some time ago that, in addition to variation relative to the septal crest, Massing and James described the variation in terms of take-off of the right bundle branch.34 The most impressive feature of the left bundle branch, in contrast to the right branch, is the marked variability found in its patterns of ramification. Its origin from the axis is broad in some individuals, and narrow in others. This can range in size from less than 1 mm to over 1 cm. As it courses down the surface of the ventricular septum towards the left ventricle, the bundle widens rapidly in some hearts, and gradually in others.34–36
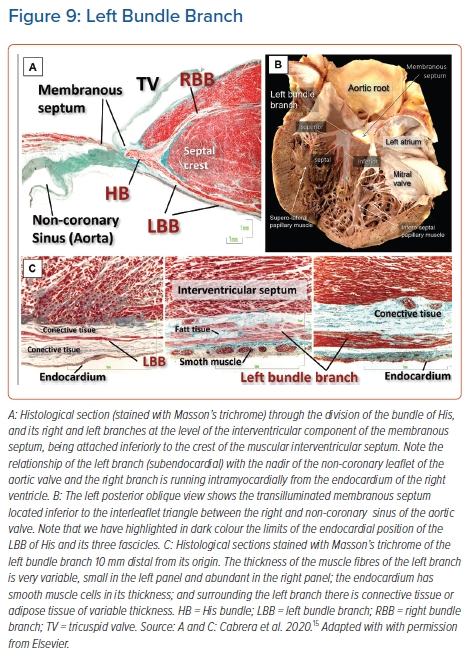
The fashion of subdivision of the left bundle continues to be debated amongst electrophysiologists. It is surprising in this regard, however, that some researchers still consider the left bundle branch in the human heart to be bifascicular.37 We are unaware of any study that endorses this investigation. Since the pioneering work of Tawara himself, it has been evident that the bundle ramifies in a trifascicular fashion.16 The stimulation data produced by Durrer et al. showed three distinct endocardial areas to be synchronously excited within the left ventricle.38 It is reasonable to presume these areas to correspond to the terminations of the three fascicles of the left bundle branch, which have been identified in the human heart by almost all anatomists. Although some have described the branching as ‘unpredictable’, our own investigations confirm that the left bundle ramifies with a thin and elongated superior radiation, a wider inferior radiation and a third radiation that covers the middle of the septum. The superior fascicle is often a thin and long structure that extends towards the superolateral papillary muscle. The inferior fascicle is usually thicker, broader and shorter. It extends towards the base of the inferoseptal papillary muscle. The septal branch emerges from the axis between the inferior and superior fascicles (Figure 9).35,39
The ideal target for direct pacing is the base of the bundle close to its origin at the septal crest. Penetration of the septal area at this level of the conduction axis should then permit excitation of the left bundle in a selective fashion. Taking account of the complexity and variability of branching, however, it is likely that the intraseptal lead may often times reach the septal branch, or even the wider inferior fascicle. It is common for the proximal portion of the branch not to be reached because of technical or anatomical difficulties. Despite this, capture of the inferior fascicle will still very often be possible. In a recent study using the 30° right anterior oblique ventricular fluoroscopic image, it was found that acceptable initial fixation points are concentrated in, or near, the area of the basal lower one-third of the ventricle. This fan-shaped area is consistent with the known anatomical location of the ramification of the inferior fascicle.40
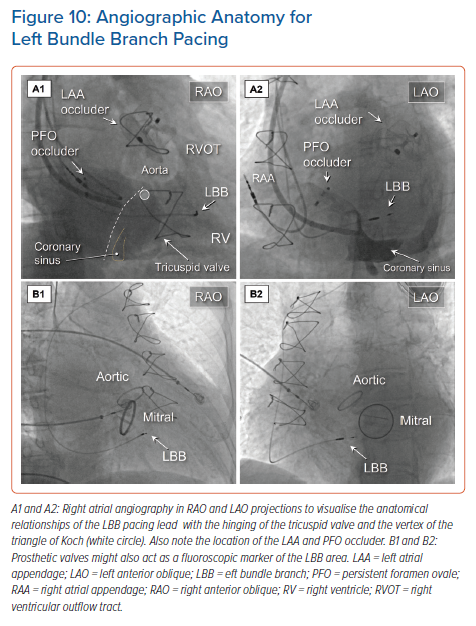
It remains challenging, but nonetheless very important, to find an acceptable initial fixation site for the pacing lead.41 Visualisation of the site of hinging of the leaflets of the tricuspid value using contrast injected into the right atrium or right ventricle may permit well-defined placement (Figure 10 and Supplementary Material Video 1). It follows that the duration of both the procedure and the time required for fluoroscopy is significantly shortened with less need to reposition the lead.42,43
During fixation of the lead, the characteristic findings of capture should be assessed using different pacing outputs. The thickness of the fascicles themselves vary, ranging from one to 25 cell layers of specialised conducting tissue, surrounded by equally varying amounts of subendocardial collagen and smooth muscle. The presence of a so-called ‘injury current’ may suggest that the tip of the lead is in close contact with the left-sided conduction system, with possible penetration into the fibrous insulating sheath and the risk of septal perforation (Figure 9).44 The large variability in the diameter, length, location, ramifications, and insulation of the bundles and their fascicles is probably the explanation behind an important degree of variability of the captured morphology when deploying the lead in anatomically small portions of septal myocardium.
Conclusion
We have reviewed, based on our own experience, the anatomical relationships and normal variability of the atrioventricular conduction axis. In particular, we have emphasised the location of the axis as it enters the subaortic outflow tract, pointing to the variability in the ramifications of the left bundle branch. These anatomical variations are of importance for the interventional electrophysiologist when seeking the optimal locations for direct pacing of either the penetrating bundle or the left bundle branch. We hope our descriptions will be prove of value for those physicians seeking to achieve permanent pacing of the atrioventricular conduction in the safest and most effective fashion.
Clinical Perspective
- There is marked inter-individual variability in the location of the penetrating atrioventricular bundle within the triangle of Koch.
- The location of site of penetration of the conduction axis is of critical importance in seeking to identify the optimal position to deploy the pacing lead if the operator is aiming to capture the fascicles of the left bundle branch from the right side via the intraseptal approach.
- Fixation points for pacing of the left bundle branch region should be concentrated in, or near, the area of the basal lower one-third of the ventricle.
- Visualisation of the site of hinging of the leaflets of the tricuspid valve, using contrast injected into the right atrium or right ventricle, may permit anatomically well-defined placement of pacing leads.