AF has major clinical implications on patients’ quality of life, morbidity with ischaemic stroke and heart failure, and mortality when compared with the general population.1 AF is the most common sustained arrhythmia and it has been calculated that it will affect 17.9 million adults in the EU and the UK by 2060.2 The increasing prevalence of AF is driven mainly by the ageing population and the high burden of risk factors and comorbidities, which raises significant issues about the use of healthcare systems and economic costs.2–4
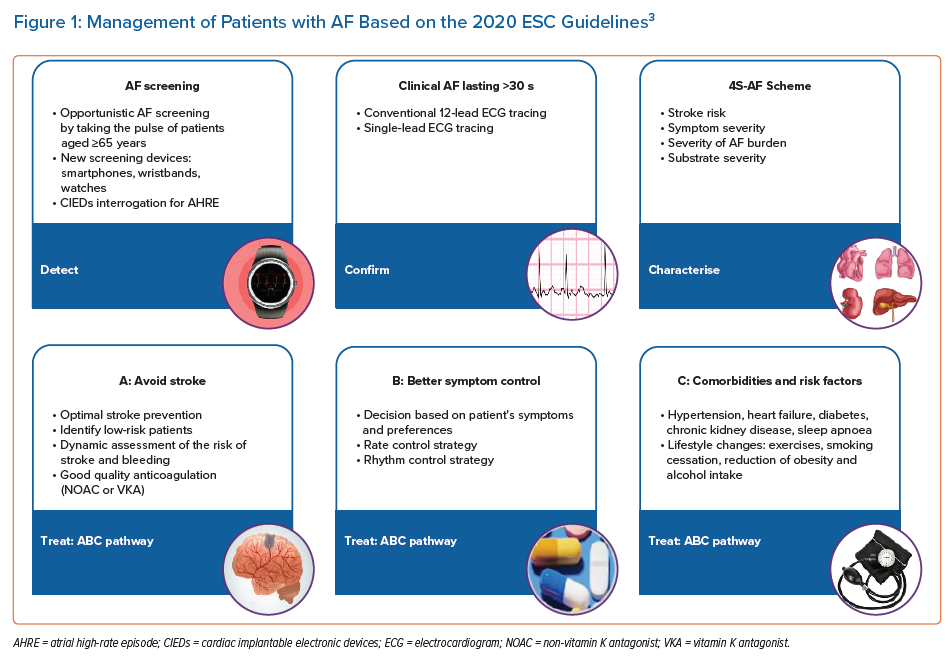
The 2020 European Society of Cardiology (ESC) Clinical Practice Guidelines for AF summarise and evaluate the available evidence from 1,492 references to provide an overview of contemporary AF diagnosis, management and research.3 Given the complexity of AF and its poorly understood mechanisms, the management of AF patients requires a holistic, multidisciplinary approach, including individual assessment, patient preferences and active involvement in decision-making. The guidelines introduce a novel, simplified, holistic approach to care for patients with AF (Figure 1), incorporating screening, diagnosis and treatment for effective, integrated management.3 These AF guidelines should consider the clinical evaluation and the choice of the best treatment strategies for each individual patient with AF.3
To accompany the guidelines, the ESC published quality indicators (17 main and 17 secondary indicators from six domains of care) to help improve and allow comparisons of the overall quality of care among AF patients at various levels, by looking at data at patient, centre or international level.3,5
Detection of AF
In recent years, substantial progress has been made in detecting AF, including asymptomatic AF. Increasing data on the identification and monitoring of AF are available for the use of wearable technology or implantable loop recorders to detect and record AF episodes.6–9 Novel tools and technologies for digital ECG analysis, in the form of wearables, machine learning and artificial intelligence, have brought potentially significant opportunities for the detection and diagnosis of AF and may be used for long-term AF screening, especially in high-risk cohorts.10 However, the diagnosis of clinical AF needs to be confirmed and documented by a conventional 12-lead ECG tracing or rhythm strip showing a typical AF pattern for ≥30 seconds. Nonetheless, a gap in data exists which raises the question of the management of patients with shorter AF duration (<30 seconds) or atrial high-rate episodes, without a ‘proper’ AF diagnosis.
Characterisation of AF
A novel pathophysiology-based characterisation of AF patients to use in daily clinical practice has been proposed in the new guidelines, summed up as the 4S-AF scheme with the four Ss being: stroke risk, symptom severity, severity of AF burden and substrate for AF.11 This structured model was proposed to support daily decision-making regarding the use of oral anticoagulation (OAC), rate or rhythm control strategy (AF ablation or antiarrhythmic drug) and the treatment of concomitant comorbidities and risk factors. It may also provide prognostic information, improving further AF management and research.3,11
Treatment of AF
The management of AF patients can be streamlined based on the AF Better Care (ABC) pathway:
- A: Avoid stroke with anticoagulation;
- B: Better symptom management with patient-centred, symptom-directed decisions on rate or rhythm control;
- C: Comorbidity and cardiovascular risk factor management, including lifestyle optimisation.12
In numerous studies, the use of a strategy that is compliant with the ABC pathway improved the outcomes for AF patients by reducing the rates of rehospitalisation, cardiovascular events and all-cause mortality.13,14 Long-term adherence and persistence with an app-based ABC pathway intervention has been demonstrated.15
A: Avoid Stroke with Anticoagulation
Effective stroke prevention with OAC is the cornerstone of the management of patients with AF and it reduces the risk of stroke and death.12 Treatment options include vitamin K antagonists (VKA) and non-VKA OACs (NOACs), whereby the NOACs are preferred.3,16,17 Maintaining a good quality of anticoagulation if VKAs are used and using label-adherent NOAC dosing is crucial. Of note, a subset of patients at high risk of stroke and bleeding have been under-represented or excluded from the randomised control trials on NOACs and special care in selecting an OAC is needed for these patients or left atrial appendage occlusion may be considered.18
Optimal stroke assessment and prevention requires a regular reassessment of stroke and bleeding risk, which is influenced by ageing or comorbidities.19,20 Risk re-evaluation should be performed within 4 to 6 months after the first visit among AF patients with a low risk of stroke (CHA2DS2-VASc = 0 in men or 1 in women). Likewise, bleeding risk is highly dynamic and a high bleeding risk score (e.g. HAS-BLED ≥3) is not a reason for withholding OAC, but these patients should have proactive management of modifiable bleeding risk factors with scheduled early follow-up and review.3,19,21
B: Better Symptom Control
Rate control allows AF to persist with well-controlled ventricular rates whereas rhythm control strategy involves the restoration of sinus rhythm using ablation, cardioversion or anti-arrhythmic drugs to mitigate AF symptoms and improve quality of life.3 Rhythm control may improve overall clinical outcomes compared with rate control in selected patient groups, for example those with heart failure or where AF has been recently diagnosed.22,23 Indeed, improvements in existing ablation techniques and tools have increased the efficacy and safety of catheter AF ablation. One recent trial showed that an early rhythm control strategy (associated with an optimised structured package of care) reduced the composite endpoint of cardiovascular-related death, stroke or hospitalisation in patients with newly diagnosed AF.23
C: Comorbidity and Cardiovascular Risk Management
Modifiable risk factors, such as weight loss, regular physical activity, smoking cessation, reducing alcohol and caffeine intake and diet modification, are often related to lifestyle choices. Subsequently, treatment of specific cardiovascular risks, such as hypertension, heart failure and coronary artery disease, and non-cardiovascular conditions, such as chronic kidney disease, diabetes and sleep apnoea, may reduce AF-related mortality and morbidity.3 Overall, strict control of risk factors, avoidance of AF triggers and treatment of underlying conditions complements stroke prevention and improves clinical outcomes by reducing AF burden or symptom severity.3
The new ESC guidelines emphasise the relevance of a tailored, holistic treatment strategy, based on the individual risk assessment and patient preferences. Shared decision-making requires educating and empowering patients by encouraging active involvement in the therapeutic process using in-depth discussion with patients and an explanation of the benefits and risks of available treatment strategies. The choice of optimal management strategy still remains a challenge, especially among AF patients at the highest risk of stroke and bleeding and requires further research.