Ventricular arrhythmias are a therapeutic challenge. They occur frequently in clinical practice, are found in patients with and without structural heart disease, and most importantly, are unpredictable and potentially deadly. Patients with a history of sustained ventricular tachycardia (VT) and VF or those at high risk for such arrhythmias, may require an ICD to prevent sudden cardiac arrest. However, despite life-saving benefit, recurrent device therapy, both appropriate and inappropriate, can have a profound psychological impact, reduce quality of life and is associated with an increase in mortality.1 About one-third of patients receive a shock from their defibrillator within 4–5 years of implantation, and 16–18% of these are inappropriate.2–4 Shock prevention includes a combination of optimised device settings as well as treatment with antiarrhythmic medication and ablation. The purpose of this paper is to review clinical trial data and propose a strategy to reduce the number of shocks in patients who require ICD implantation to prevent sudden cardiac death, with a focus on the treatment of ventricular arrhythmias. The specific treatment of narrow complex tachycardias such as AF and atrial flutter is beyond the scope of this review.
Optimal Device Programming
Optimal device programming minimises the occurrence of device therapy, improves quality of life and, in many cases, improves mortality rates. As seen below, many device parameters have been evaluated to achieve these goals. An additional perspective on optimal device settings is provided in an excellent review by Spragg and Berger.5
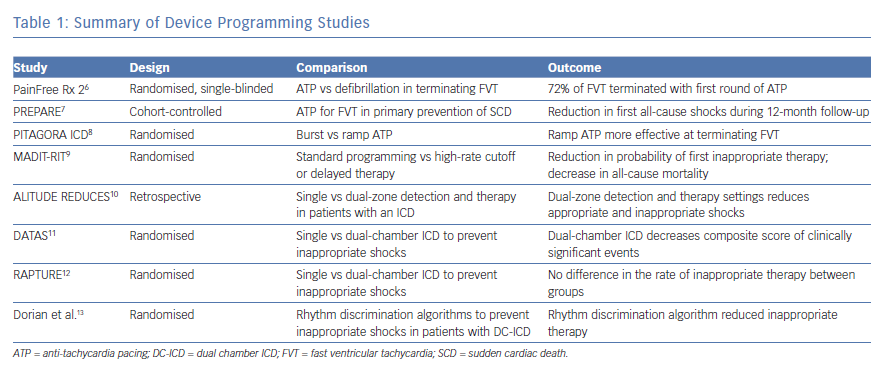
Device therapy with antitachycardia pacing (ATP) improves the efficiency of ICD function by decreasing the incidence of ICD delivered shocks. The Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (PainFREE Rx 2) trial randomised patients to receive either ATP or defibrillation for fast ventricular tachycardia (FVT) (188–250 bpm). Patients included in the study were diagnosed with both ischaemic and non-ischaemic cardiomyopathy and had ICDs implanted for either primary or secondary prevention. Primary prevention criteria included ICD implantation in patients without a prior diagnosis of VF, sustained VT or a combination of unexplained syncope with inducible VT. The trial data found that 72% of FVT could be terminated with the first attempted ATP. Patients treated with ATP compared to shock had an improvement in both mental and physical quality of life scores.6
The Primary Prevention Parameters Evaluation (PREPARE) trial authors studied the strategy of ATP to terminate FVT in patients requiring an ICD for primary prevention. The investigators of this study performed a prospective cohort-controlled trial of 700 patients, using patients from the Comparison of Empiric to Physician-Tailored Programming of Implantable Cardioverter Defibrillators Trial (EMPIRIC) and Multicenter InSync Implantable Cardioversion Defibrillation Randomized Clinical Evaluation (MIRACLE ICD) trials as controls. They found that treating patients with one sequence of ATP before defibrillation in an FVT zone between 182–250 bpm reduced the number of patients receiving a first all-cause shock within the first 12 months from 17% in the control group to 9% in the study population and decreased mortality in the study group.7
Antitachycardia pacing delivery method was analysed in the Randomized Study to Compare Ramp Versus Burst Antitachycardia Pacing Therapies to Treat Fast Ventricular Tachyarrhythmias in Patients With Implantable Cardioverter Defibrillators (PITAGORA ICD) trial. In this study, the investigators randomised 206 patients with both ischaemic and nonischaemic cardiomyopathy as well as those with ICD for both primary and secondary prevention to either ramp or burst ATP as an initial therapy for FVT. The investigators found that 54% of FVT episodes were successfully treated in the ramp arm versus 75% of FVT episodes in the burst arm, providing evidence that burst-style ATP is more effect than ramp-style.8
The investigators in the Multicenter Automatic Defibrillator Implantation Trial: Reduce Inappropriate Therapy (MADIT-RIT) trial studied the effects of limiting device therapy to a high rate cutoff or delaying therapy at slower rates. The trial randomised 1500 patients to three arms that compared standard device programming to programming a high-rate VT detection zone greater than 200 bpm before delivery of device therapy, programming with a 60-second delay for VT greater than 170 bpm or a 12-second delay at 200 bpm before delivery of device therapy. The primary endpoint was first occurrence of inappropriate device therapy. Secondary endpoints included death from any cause or first episode of syncope. Patients with programming that included a high rate cutoff or a delay to therapy had a lower cumulative probability of first inappropriate therapy as well as a decrease in all-cause mortality. The hazard ratio of first occurrence of inappropriate therapy and death in the high-rate versus conventional therapy was 0.21 and 0.45, respectively. The hazard ratio for the same parameters in the delayed versus conventional therapy was 0.24 and 0.56, respectively.9
While the MADIT trial did not directly evaluate the effects of dual-zone detection and therapy settings, the results of the study implied that dual-zone therapy settings reduced inappropriate shocks. This observation was previously studied in the ALTITUDE Real World Evaluation of Dual-zone ICD and CRT-D Programming Compared to Single-zone Programming (REDUCES) study. In this retrospective study, the authors reviewed device data in patients who received single-chamber, dual-chamber and dual-chamber, biventricular ICDs who enrolled in the Boston Scientific LATITUDE remote monitoring program. Patients were grouped based on the parameters of single or dual-zone detection and therapy at detection rates of ≤170 bpm, 170–200 bpm, or ≥200 bpm. The primary endpoint in this analysis was time from ICD implantation until the first occurrence of ICD therapy or death. Patients programmed with dual-zone detection and therapy parameters had a significant decrease in both all-cause and inappropriate shocks in the detection rate groups of ≤170 bpm and 170–200 bpm. There was a trend towards decreased all-cause and inappropriate shocks in the ≥200 bpm rate detection group. They also noted that atrial rhythms were the cause of the majority of shocks occurring at rates below 180 bpm.10
Given the ability of dual chamber devices to monitor rhythms in both the atria and ventricles, studies were designed to test the hypothesis that dual-chamber devices could prevent inappropriate therapy via rhythm discrimination. The Dual Chamber and Atrial Tachyarrhythmias Adverse Events Study (DATAS) trial randomised 334 patients with an ACC/AHA Class I indication for a single chamber (SC)-ICD to one of three arms: SC-ICD, DC-ICD or a dual chamber (DC)-ICD programmed as SC-ICD, termed a simulated SC-ICD. The primary endpoint was a composite of five predetermined clinically significant adverse events: all-cause mortality, invasive intervention due to cardiovascular cause, hospitalisation greater than 24 hours or prolongation of hospitalisation due to cardiovascular cause, inappropriate shocks and sustained symptomatic atrial tachycardia that required urgent termination or lasted more than 48 hours leading to therapeutic intervention. The authors developed a scoring system based upon the number of clinically significant events the patient experienced during the study period. They concluded that patients with a DC-ICD had a lower rate of clinically significant events compared to patients randomised to receive a SC-ICD. However, the study was not powered to make statistical comparisons for any single component of the primary endpoint and could not make conclusions on how implantation of a DC-ICD directly affected rates of inappropriate therapy or mortality.11 The Reduction And Prevention of Tachyarrhythmias and Shocks Using Reduced Ventricular Pacing with Atrial Algorithms Study (RAPTURE) trial compared the rate of inappropriate therapy in patients with a dual-chamber ICD to those with a single-chamber ICD. The authors randomised 100 patients who met indications for primary prevention to either a dual or single chamber ICD. The primary endpoint was the proportion of patients receiving an inappropriate shock within the first 12 months after ICD implantation. During an average follow-up of 12.0 ± 2.6 months, there was no statistical difference in the proportion of patients receiving inappropriate therapy between groups.12
While there has been no consistent data to suggest that upgrading to a DC-ICD from a single chamber device for the purpose of rhythm discrimination is beneficial to patients, there is data to suggest that rhythm discrimination algorithms programmed into dual-chamber devices in patients who require pacing for diagnoses, such as sinus node dysfunction or conduction disease has improved shock prevention. In a study by Dorian et al., 149 patients with a DC-ICD and a history of sustained VT or VF were randomised to either an enhanced therapy group or rate-only control group. The patients followed-up at regular intervals of 3, 6 and 12 months, if the patient was symptomatic or received device therapy. The primary endpoint was the time to first inappropriate therapy. The primary endpoint occurred less frequently in the enhanced therapy group resulting in a hazard ratio of 0.468 (95% CI [0.266–0.822]), reflecting a 53.2% reduction in the risk of inappropriate therapy (p = 0.011).13 Table 1 summarises the device programming trials.
Medical Therapy
Medical prevention of ICD shock begins with optimal treatment of the underlying medical condition. Heart failure should be corrected using guideline-directed medical therapy being careful to monitor volume and electrolyte concentrations. Patients with chronic systolic heart failure should be continuously evaluated for cardiac resynchronisation therapy. Patients with coronary artery disease should be evaluated for active ischaemia. Treatment of hypertension and other important medical conditions such as diabetes must be optimised. While no pharmacological agent is currently labelled to prevent recurrent ICD therapy, several antiarrhythmics have been studied to determine their value in reducing ICD shocks and pace termination. Below is a summary of studies examining potential medications to reduce shocks due to ventricular arrhythmias. Another perspective on this topic is provided in an excellent review by Abboud and Ehrlich.14
Sotalol
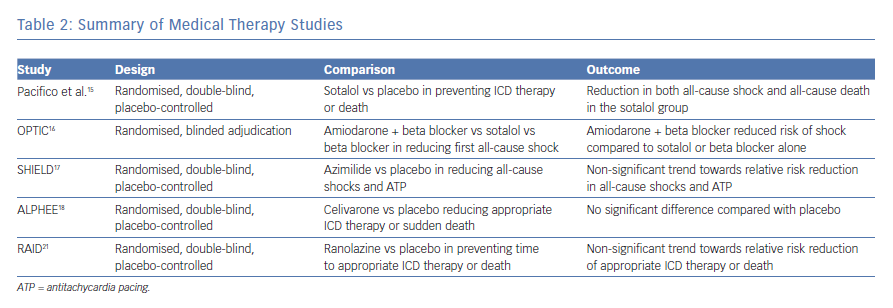
Sotalol, a beta-blocker with class III antiarrhythmic properties, was studied in patients who received an ICD for secondary prevention for its efficacy in preventing shocks and death. Patients were randomly assigned to placebo or sotalol treatment and were followed for 12 months. Patients who were randomised to sotalol were initiated on 120 mg twice daily; however, this dose could be adjusted to a minimum of 80 mg or a maximum of 160 mg twice daily depending on efficacy and side-effects of the drug. The primary endpoints of the study were death from any cause or shock for any reason. Significantly fewer patients treated with sotalol reached the primary endpoint compared with the placebo.15 Despite this benefit, sotalol can induce bradycardia in patients on concomitant beta-blocker therapy, limiting its use and creating the need for alternative medical options.
Amiodarone
Investigators in the Optimal Pharmacological Therapy in Cardioverter Defibrillator Patients (OPTIC) trial compared treatment with sotalol to combination therapy with amiodarone and beta-blocker. They randomised 412 patients with a history of sustained VT, VF or cardiac arrest as well as an ejection fraction (EF) of less than 40% to receive sotalol, beta-blocker or amiodarone plus beta-blocker. Patients receiving sotalol received 240 mg per day in divided doses if their creatinine clearance was above 60 ml/min. If their creatinine clearance was between 30–60 ml/min, this dose was reduced to 160 mg per day. Patients receiving amiodarone were loaded with 400 mg twice per day for two weeks, followed by 400 mg per day for four weeks and then treated with 200 mg per day until the end of the study. Patients randomised to the beta-blocker arm or amiodarone arm were treated with either metoprolol 100 mg per day, carvedilol 50 mg per day or bisoprolol 10 mg per day. Patients were followed for 12 months with the primary outcome being first occurrence of any shock. Patients treated with the combination therapy of amiodarone and beta-blocker had a reduced risk of shock compared to beta-blocker use alone or sotalol use alone. The hazard ratio comparing the combination treatment to beta-blocker alone or sotalol alone was 0.27 and 0.43, respectively. When sotalol was compared to beta-blocker, there was a trend towards reduced risk of shock; however, this result was not significant.16
Azimilide
Azimilide, a class-III agent, has also been studied to reduce recurrent ICD therapy. Authors of the Shock Inhibition Evaluation with Azimilide (SHIELD) trial recruited patients with an ICD who had spontaneous, sustained VT or patients who experienced cardiac arrest and had an EF of ≤40% and randomised them to placebo, 75 mg or 125 mg of azimilide. The two primary endpoints in the study were (1) the combined incidence of all-cause shocks plus symptomatic tachyarrhythmias terminated by ATP and (2) all-cause shocks. Patients receiving azimilide had a dose-dependent relative risk reduction of the first primary endpoint of 57% and 47%, respectively, as well as a dose-dependent relative risk reduction of the second primary endpoint of 28% and 17%, respectively; however, none of these values reached statistical significance.17
Celivarone
Celivarone, an antiarrhythmic with class I, II, III and IV properties was also studied to reduce the recurrence of ventricular tachycardia. The authors of the Dose Ranging Study of Celivarone with Amiodarone as Calibrator for the Prevention of Implantable Cardioverter Defibrillator Interventions or Death [ALPHEE] study designed a multiple dose, randomised, double blind, placebo-controlled trial with amiodarone as a calibrator. Patients with a left ventricular EF of ≤40% as well as an ICD for primary prevention with at least one ICD intervention for VT/VF in the previous month, as well as patients with an ICD for secondary prevention with ICD implantation in the previous month or at least one ICD intervention for VT/VF were randomised. They received once-daily therapy for 6 months with placebo or celivarone 50, 100 or 300 mg, or amiodarone 600 mg for 10 days followed by 200 mg daily thereafter. The primary efficacy endpoint was the occurrence of VT/VF- triggered ICD interventions (shocks or antitachycardia pacing) or sudden death, analysed with a time to first event approach. The hazard ratio for the primary endpoint comparing the study drug to placebo ranged from 0.86 for the celivarone 300-mg group to 1.199 for the celivarone 50-mg group; however, none were statistically significant.18
Ranolazine
Ranolazine is an antianginal and anti-ischaemic drug with possible antiarrhythmic properties via late sodium channel blockade. Ranolazine reduced episodes of VT lasting at least eight beats in the first week of rhythm monitoring after admission for an acute coronary syndrome.19,20 These data sparked interest in the drug’s ability to reduce appropriate ICD therapy. The Ranolazine in High-Risk Patients with Implanted Cardioverter-Defibrillators (RAID) trial was a double blind, placebo-controlled trial evaluating the efficacy of ranolazine in treating recurrent VT/VF or death in patients with ischaemic and non-ischaemic cardiomyopathy. The patient population consisted of high-risk patients with an ICD or cardiac resynchronisation therapy with defibrillator (CRT-D) for secondary prevention with documented VT, VF or cardiac arrest, or primary prevention patients regardless of date of device implantation with an ejection fraction of ≤35%. Patients had at least one of the following high-risk criteria: blood urea nitrogen of ≥26 mg/dl, QRS duration of ≥120 milliseconds, documented paroxysmal or permanent atrial fibrillation, non-sustained VT or >500 premature ventricular contractions on Holter monitoring. Patients were randomly assigned ranolazine or placebo in a 1:1 fashion. Patients were started on 500 mg twice daily for one week with an increase in dosage to 1,000 mg twice daily if the drug was tolerated. The primary endpoint of the study was a composite consisting of the time to VT or VF (requiring ATP therapy or ICD shock), or death, whichever occurred first. In the pre-specified intention-to-treat analysis, the primary endpoint of VT or VF requiring ICD therapy (ATP or shock), or death, occurred in 174 patients (34.1%) in the ranolazine arm and in 198 (39.4%) in the placebo arm (HR: 0.84; 95% CI [0.67–1.05]; p=0.117). While the results did not reach statistical significance, the study was underpowered based on pre-specified statistical criteria. In a pre-specified secondary analysis, patients randomised to ranolazine had a marginally significant lower risk of ICD therapies for recurrent VT or VF (hazard ratio: 0.70; 95% confidence interval: 0.51–0.96; p=0.028).21 See Table 2 for a summary of medical therapy studies.
Ablation Therapy
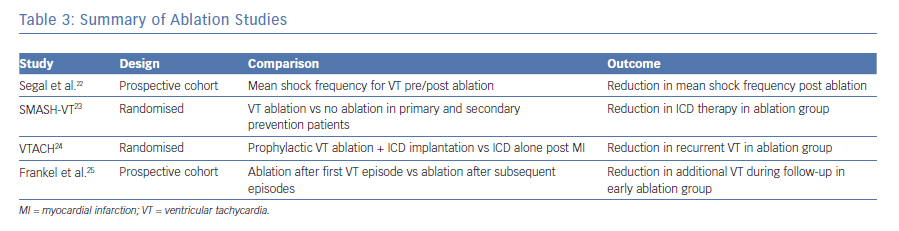
Multiple studies have provided evidence for the effectiveness of ablation to reduce the recurrence of ventricular tachycardia in patients with structural heart disease. A study published by Segal et al. in 2005 reported the results of catheter ablation for myocardial infarction-related VT in a group of 40 patients who were followed for 24 ± 18 months. These patients underwent targeted VT ablation with the mean shock frequency post ablation reduced from 6.8 ± 7.3 per month in the year prior to ablation to 0.05 ± 0.12 per month after ablation, with over 24.7 ± 18.9 months of follow-up (p<0.0001).22 The authors of Substrate Mapping and Ablation in Sinus Rhythm to Halt Ventricular Tachycardia (SMASH-VT) studied the ability of ablation therapy to reduce VT in patients with an ICD for secondary prevention as well patients with an ICD for primary prevention who subsequently received an appropriate shock. They randomised 128 patients to receive targeted ablation versus no additional therapy. The patients were then followed for up to 24 months post ablation. Results of the study showed that 12% of the ablation group received ICD therapy (shock or ATP) versus 31% of the control group.23 Despite the improved rates of ICD therapy, the results of the SMASH-VT trial reinforce the fact that patients with structural heart disease should still receive an ICD given the limited efficacy of ablation.
The Ventricular Tachycardia Ablation in Coronary Heart Disease (VTACH) study investigators evaluated prophylactic VT ablation in patients with a history of myocardial infarction, stable clinical VT (defined as a VT not leading to cardiac arrest or syncope and during which the systolic blood pressure was higher than 90 mmHg) and an ejection fraction under 50%. Patients were randomised to ablation plus ICD implantation or ICD implantation alone and were followed for approximately 2 years (22.5 months). At follow-up, fewer patients in the ablation group experienced recurrent VT/VF; 47% of patient in the ablation group did not experience recurrent VT/VF versus only 29% for the ICD only group. However, this benefit was only manifest in patients with an ejection fraction above 30%.24
Frankel et al. completed a prospective cohort study to evaluate the timing of VT ablation. Their data suggested that if ablation for VT is considered, the procedure should be completed earlier in the course of disease. They followed 98 consecutive patients with structural heart disease referred to their centre for VT ablation. Patients were stratified into early and late referral, meaning those patients referred after a first episode of VT versus experiencing two or more episodes. The results of their study showed that 75% of patients referred early for VT ablation remained free of additional episodes in the following year, versus 50% of patients referred late.25 See Table 3 for summary of ablation studies.
Hybrid Therapy
While the goal of ablation for some patients may be to discontinue use of antiarrhythmic therapy, medical management and catheter ablation may be pursued as a dual strategy. In studies of patients with cardiomyopathy who underwent VT ablation for VT/VF, the number of patients with recurrence of VT during follow-up was related to withdrawal of antiarrhythmics; 68% of patients who had medication changes had recurrent VT compared to 41% of patients who did not have medications changes following ablation in the follow-up period.26 A meta-analysis of randomised controlled trials confirmed that both catheter ablation and antiarrhythmic drugs reduce the number of appropriate shocks, while antiarrhythmic drugs decreased the number of inappropriate shocks. However, a comparison between catheter ablation and use of antiarrhythmic drugs did not show a reduction of recurrent VT relative to the other.27
The 2016, the Ventricular Tachycardia Ablation versus Escalated Antiarrhythmic Drug Therapy in Ischemic Heart Disease (VANISH) trial compared escalating the dose of antiarrhythmic therapy versus catheter ablation using the primary endpoints of composite death, VT storm or appropriate ICD shock. Patients with ischaemic cardiomyopathy and an ICD who experienced ventricular tachycardia despite antiarrhythmic therapy were randomised to increasing doses of antiarrhythmic therapy or catheter ablation. The dose of amiodarone administered to patients assigned to the escalation arm was based on baseline medical therapy. If the patient was on antiarrhythmic therapy other than amiodarone, the patient was initiated on 400 mg twice-daily amiodarone for two weeks, followed by 400 mg daily for four weeks, then 200 mg daily thereafter. If the patient was currently on a dose of amiodarone less than 300 mg daily, the patient was treated with a loading dose of 400 mg twice daily of amiodarone for two weeks followed by 400 mg daily for one week, then 300 mg daily thereafter. If the patient was currently taking at least 300 mg daily of amiodarone, their current dose was continued and mexiletine was added at a dose of 200 mg, three times daily. The patients assigned to ablation therapy underwent a procedure that followed a standardised approach that specifically targeted all inducible ventricular tachycardias. Patients who received ablation had a lower rate of the primary outcome of death, VT storm or appropriate ICD shock. The primary outcome occurred in 59.1% of patients in the ablation group and 68.5% of those in the escalated-therapy group (hazard ratio in the ablation group, 0.72; 95% CI [0.53–0.98]; p=0.04). In a subgroup analysis, patients treated with a baseline regimen of amiodarone had a lower incidence of the primary outcome (hazard ratio 0.55; 95% CI [0.38–0.80]; p=0.001). Patients who were not on amiodarone at baseline but were initiated on the drug as a part of the trial showed no difference in the primary outcome of the composite of death, VT storm or appropriate ICD shock as compared to patients treated with VT ablation.28
Conclusion
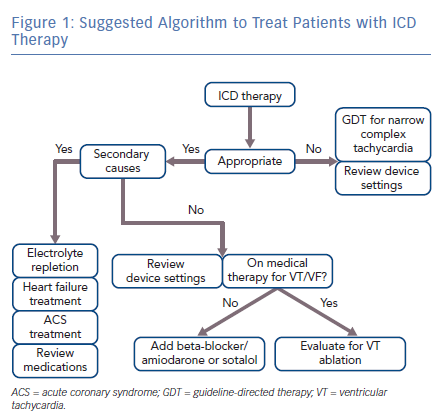
Minimizing recurrent ICD shocks will be dependent on optimal ICD programming, medical therapy and strategic ablation. Figure 1 suggests an algorithm to guide management. If a patient experiences a defibrillation, the underlying rhythm should be analysed to determine if the device therapy was appropriate or inappropriate. Narrow-complex tachycardias such as atrial fibrillation or flutter should be managed according to current guidelines. Inappropriate shocks caused by narrow complex tachycardias can be reduced with higher rate thresholds. Specific rate cut-off settings should be adjusted to the clinical context; however, the data suggests that the majority of atrial tachycardias occur at rates below 180 bpm. Appropriate shocks can be minimised by including dual-zone therapy programming, burst-ATP before attempted defibrillation of FVT and time-delay before device therapy, as many episodes of VT will spontaneously terminate. Patients with dual-chamber devices would benefit from programming rhythm discrimination algorithms to reduce frequency of inappropriate shocks; however, there is not enough evidence to support upgrading from a single chamber ICD to a dual chamber ICD for the sole purpose of rhythm discrimination. A discussion of rhythm discrimination algorithms is provided in a review by Spragg and Berger.5 A list of rhythms causing inappropriate shocks is provided in Table 4.
Evaluate the patient for secondary causes of VT/VF including, but not limited to, medication effect, electrolyte depletion, acute heart failure or active ischemia. Chronic systolic heart failure patients should be evaluated for cardiac resynchronisation therapy. If there are no underlying aetiologies, or the patient continues to experience recurrent VT/VF despite correction of underlying aetiologies, VT/VF can be minimised by treatment with the combination therapy of amiodarone and a beta-blocker. Amiodarone dosing includes an initial loading period followed by a maintenance period. Patients in the trials noted above, who were not previously taking amiodarone received 400 mg twice per day for 2 weeks, followed by 400 mg once per day for 4 weeks, followed by 200 mg per day for the remainder of the trial period as maintenance dosing. If the patient is intolerant of amiodarone, sotalol can be used as a second-line therapy. Patients treated with sotalol can receive a maximal dose of 160 mg twice per day; however, sotalol dosing may need to be decreased based on tolerance of side-effects, QT interval prolongation or renal function. Additionally, potential candidates for sotalol may be limited given the high prevalence of beta-blocker use for comorbid conditions. If VT/VF persists, or the patient is intolerant of medical therapy, the patient should be evaluated for targeted radiofrequency ablation of the focus of VT/VF. Given current data, proceeding with VT ablation earlier in the course of disease may be beneficial as opposed to intensifying medical therapy. Patients on medical therapy who undergo ablation for VT/VF would likely benefit from remaining on medical therapy post-ablation if tolerated. Ultimately, the decision to treat a patient with ablation versus medical therapy will depend upon the patient’s comorbidities as well as their preference and tolerance of procedural risk versus medication side-effects.
Clinical Perspective
This review will provide insight and advice for physicians caring for patients with recurrent ICD therapy due to ventricular arrhythmias in the following areas:
- Adjustment of ICD setting
- Adjustment or addition of specific medications
- Timing of VT ablation