Catheter ablation is an effective strategy to maintain sinus rhythm in patients with symptomatic atrial fibrillation (AF), which has evolved from a highly specialised technique to a first-line therapy.1–3 The cornerstone of ablation is pulmonary vein isolation (PVI).4 Over the last decade, ablation devices have undergone technical improvements, aiming for better lesion durability and ablation outcomes. However, significant complications have been reported in survey studies and patient safety remains of concern.4–9 Although operators have become more experienced, technical advances with improved energy transfer may increase procedural risk. As a consequence, catheter design and ablation protocols have been adapted to prevent complications. For individualised patient care and device selection, knowledge of potential risks and benefits for the different available devices is important. The aim of this review is to provide an overview of type and incidence of complications and strategies for prevention for single-tip and multi-electrode radiofrequency catheter ablation (RFCA) and balloon-based ablation devices.
Point-by-Point Radiofrequency Ablation
After evidence that the pulmonary veins (PVs) are the primary source of AF,10,11 non-cooled radiofrequency ablation of ectopic beats from the PVs has been introduced.12,13 Due to the high incidence of PV stenosis,14 ablation has evolved from segmental ablation of the PVs guided by a circular mapping catheter4,15,16 to wide-area circumferential PV isolation.17
Historical Overview
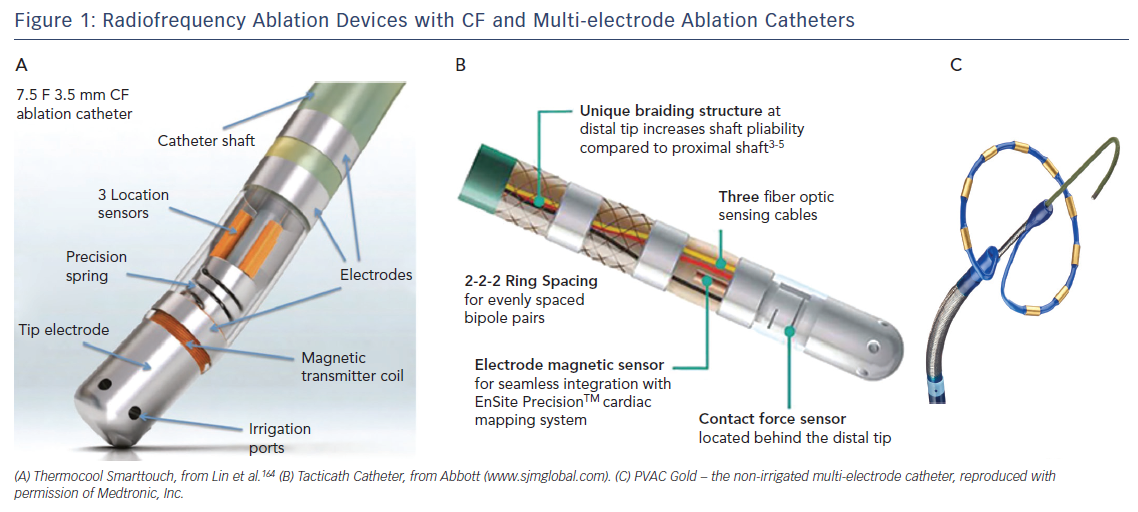
Catheter irrigation resulted in a lower risk for coagulum formation, allowing for higher energy transfer with larger and deeper lesions18,19 and improved outcome,20 with a current AF free survival of 46–94 % at 1-year follow-up (Table 1a/Table 1b). The introduction of three-dimensional electro-anatomical mapping systems (CARTO, Biosense Webster Inc., Diamond Bar, CA, USA and Ensite, Abbot, St Paul, MN, USA) and image-integration tools has been associated with improved efficacy.21–25 Contact-force (CF) measurement during ablation has been developed to improve lesion formation (Thermocool Smarttouch, Biosense and Tacticath, Abbot; Figure 1) with a reported one-year AF free survival between 52 and 94 % (Table 1a/Table 1b). There are conflicting reports whether CF improves ablation outcome (Table 1b),26,27 suggesting that CF parameters need to be validated.26 Data from a recent meta-analysis suggest that ablation guided by CF is associated with improved median outcome at 12-months follow-up.28 Recent developments focus on improved near-field resolution by combining recordings from large-tip electrodes with recordings from micro-electrodes (QDOT-micro technology for Biosense Webster Inc.).
Procedure Time
Procedural length has been associated with higher complication rates.29 Although radiation exposure can be reduced with 3D mapping systems,24 point-by-point ablation often requires longer procedure times compared with single-shot techniques. Reported mean procedural times range between 101 and 284 minutes (Table 1a/Table 1b). Contact-force has been associated with reduced procedure, ablation and fluoroscopy times28 and high-power-short-duration radiofrequency applications to further reduce procedure time are currently under investigation.30–32 Fluoroscopy time for RFCA, however, approaches to zero under increasing experience of 3D mappings systems and intracardiac electrocardiography.33,34
Complications
The use of image integration and electro-anatomical mapping has been associated with fewer complications.20–24,35,36 Whether CF-guided ablation improves safety requires additional investigation. In a recent meta-analysis, the overall complication and tamponade rates were 3.8 % and 0.5 % for CF and 3.9 % and 0.9 % for non-CF ablation.28 Irrigated catheters (Thermocool™, Biosense and Coolpath™, Abbot) have been introduced to prevent endothelial charring in particular at sites with low blood flow.19 Indeed, with irrigation, less micro-embolic signals have been detected with trans-cranial Doppler.37 Advanced irrigation technology (Thermocool Surround Flow and Abbot FlexAbility) reduces irrigation volume with maintenance of the safety profile.38 Thromboembolic event rates (stroke and transient ischaemic attack) range between 0.2 and 1 % for irrigated catheters. Phrenic nerve palsy (PNP) is rare (0.01–0.6 %) and mainly transient. Similar, the reported incidence of oesophageal and vagal injury is low, ranging between 0.05 and 0.5 % (Table 1). However, a study focusing specifically on gastrointestinal complications reported an 11 % incidence of thermal oesophageal lesions and a 17 % incidence of gastroparesis.39 In the Manufacturer and User Facility Device Experience database of 2689 ablations, the incidence of atrial-oesophageal fistula as a percentage of all reported complications for CF catheters was higher (5.4 %: 65 of 1202 cases) compared with non-CF catheters (0.9 %: 13 of 1487 cases).40 These numbers do not reflect the absolute incidence however. Pulmonary vein stenosis (PVS) after CF-guided ablation was only reported in one study with an incidence of 0.7 %.41
Multi-electrode Catheters
Historical Overview
Multi-electrode RF catheters have the potential to reduce ablation and procedural time. The pulmonary vein ablation catheter (PVAC, Medtronic, Minneapolis, MN, USA) can deliver RF energy in different duty-cycled unipolar/bipolar modes. One-year AF-free survival off AAD with the first-generation device was 61 % in patients with paroxysmal AF.42 To reduce the embolic risk potentially associated with non-irrigated RF catheters, submerging the catheter in saline before introduction and maintaining an activated-clotting time (ACT) above 350 seconds have been recommended. As interaction of electrodes 1 and 10 was associated with occurrence of asymptomatic cerebral embolism,43 the current generation catheter (PVAC-Gold; Figure 1) has only nine electrodes with a larger inter-electrode spacing and different electrode composition (from platinum to gold) for better heat conductivity. Reported one-year AF free survival with PVAC-Gold ranges from 60 to 71 %.44–46 Studies comparing the efficacy of PVAC and PVAC-Gold found no significant difference at 1-year follow-up (64–65 % and 68–70 %, respectively).45,47 Other (irrigated) multi-electrode catheters in the past were withdrawn because of safety concerns (e.g. new multipolar irrigated radiofrequency ablation catheter, Biosense Webster Inc., Multi-array septal catheter/Multi-array ablation catheter, Medtronic Inc. and High Density Mesh ablator, Bard Electrophysiology, Lowell, MA, USA).48
Procedure Time
Ablation with a smaller number of simultaneously activated electrodes to reduce thrombo-embolic risk has significantly prolonged procedure times (159±39 versus 121±15 minutes) with the first generation PVAC.49 For the PVAC-Gold catheter shorter procedure times (94–117 minutes) have been reported.45,47
Complications
Asymptomatic cerebral embolisms were significantly higher with PVAC (incidence 38–39 %) than with irrigated RFCA and cryoballoon ablation.50–53 The potentially high embolic risk is supported by studies on micro-embolic signals recorded with transcranial Doppler ultrasonography.54–56 However, after technical modifications to eliminate electrode 1–10 interactions, the duration of micro-embolic signals was reduced with only 33 %.57,58 The clinical relevance of asymptomatic cerebral embolism detected on MRI and trans-cranial Doppler remains, however, unclear.59,60 Despite technical improvements, the second-generation PVAC-Gold catheter still showed a high incidence of asymptomatic cerebral embolism (20 % versus none, p=0.011) and a higher amount and duration of micro-embolic signals compared with irrigated RFCA in a randomised clinical trial from our centre.58 PNP is uncommon after PVAC ablation. It was first reported in 201061 and occurred in only 1/272 (0.4 %) consecutive patients.62 PVAC ablation is usually performed at the ostium of the PVs and a detectable narrowing of the PV diameter has been reported in 23 % of patients and 7 % of veins.14,63,64
Balloon-based Devices
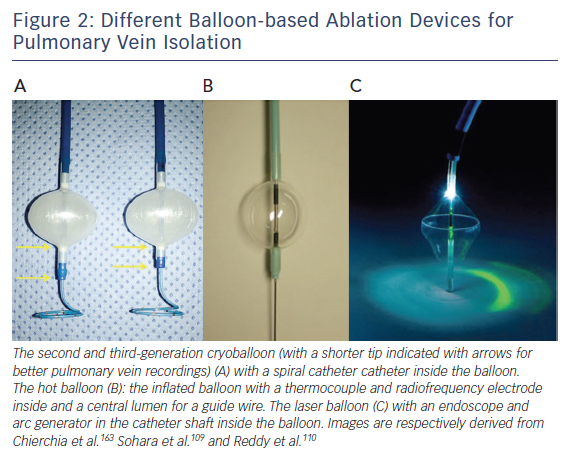
Several balloon-based devices have been developed for PVI (Figure 2), including the cryoballoon, the hotballoon, the endoscopic laserballoon and the high-intensity focused ultrasound balloon. The latter is no longer available (for safety reasons) and will not be discussed in this review. A potential limitation of these devices is the more distal PVI compared with point-by-point isolation.65 However, over the last decade, balloon-based devices have undergone important technical improvements.
Cryoballoon
Historical Overview
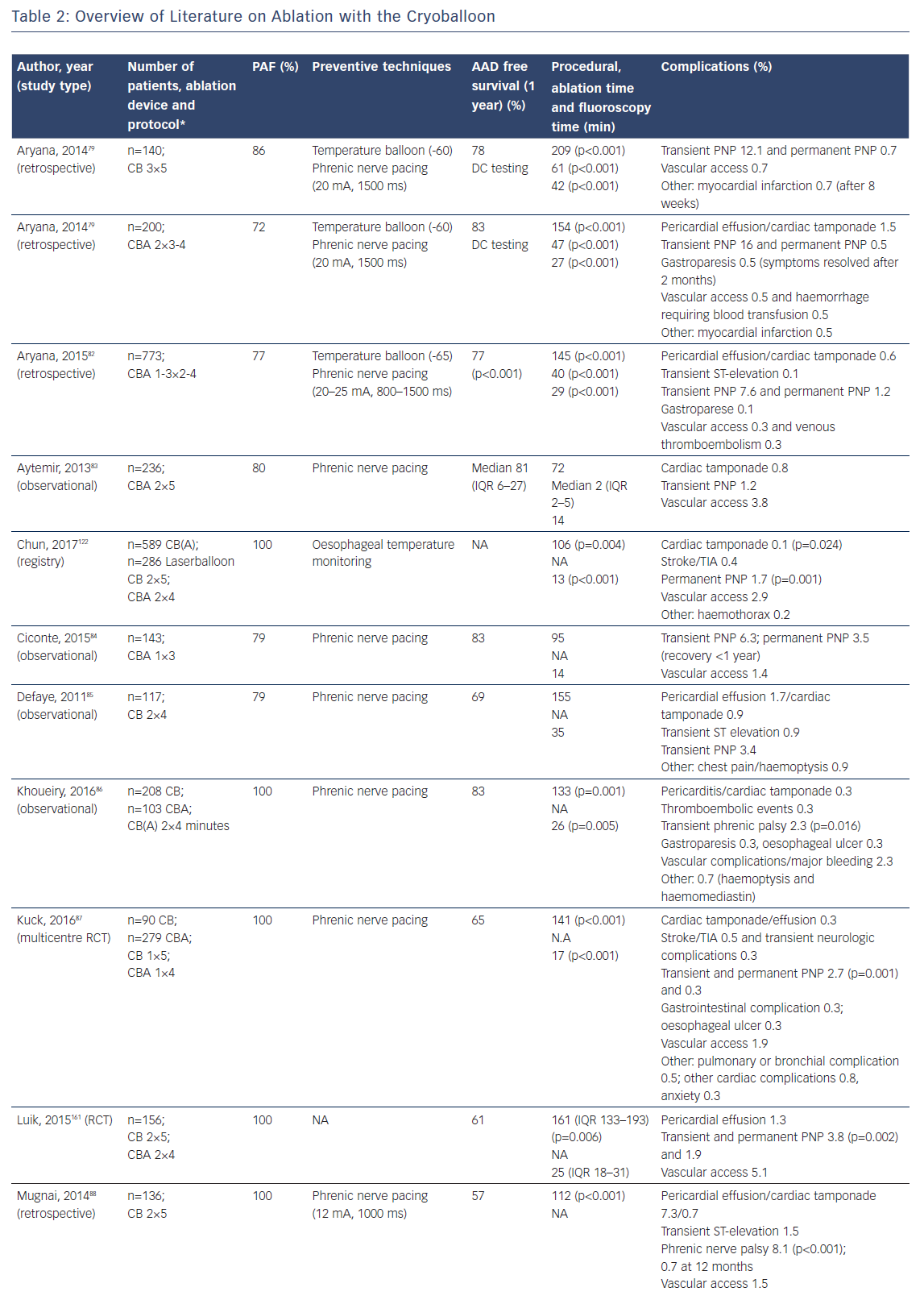
First animal studies with cryoballoon ablation were published in 2005.66,67 A double-lumen balloon is cooled by expansion of NO2.66 The second-generation cryoballoon (Arctic Front Advance, Medtronic Inc., Minneapolis, MN, USA) has an increased gas flow, improved temperature uniformity and a more proximal cooling of the balloon with more internal injection ports compared with the first-generation.68 The broader cooling zone, together with easier positioning of the balloon with the second-generation steerable sheath (Flexcath Advance) and real-time assessment of PV isolation with the intraluminal spiral catheter (Achieve) has resulted in enhanced lesion durability and more antral ablation.69,70 Recent studies reported success rates (off AAD) of 76–86 % after 1–2 years for the first and second generation cryoballoon (Table 2).71–78 Freedom of AF off drugs was reported in 48–74 % of patients for the first-generation cryoballoon and in 65–83 % for the second-generation cryoballoon at 1-year follow-up. In a retrospective study comparing the two balloons, no significant differences in outcome were observed (78 versus 83 % at 1-year follow-up).79 The third-generation cryoballoon with a shorter tip to facilitate better PV-signal recordings is still being developed.
Procedure Time
With the development of the second-generation cryoballoon, the ablation protocol has been adapted with reduced cryo-application times (180 seconds instead of two-times 300 seconds).79,80 Recent studies evaluating shorter application times based on the time-to-isolation showed a similar efficacy at 1-year follow-up.72–77, 81
Complications
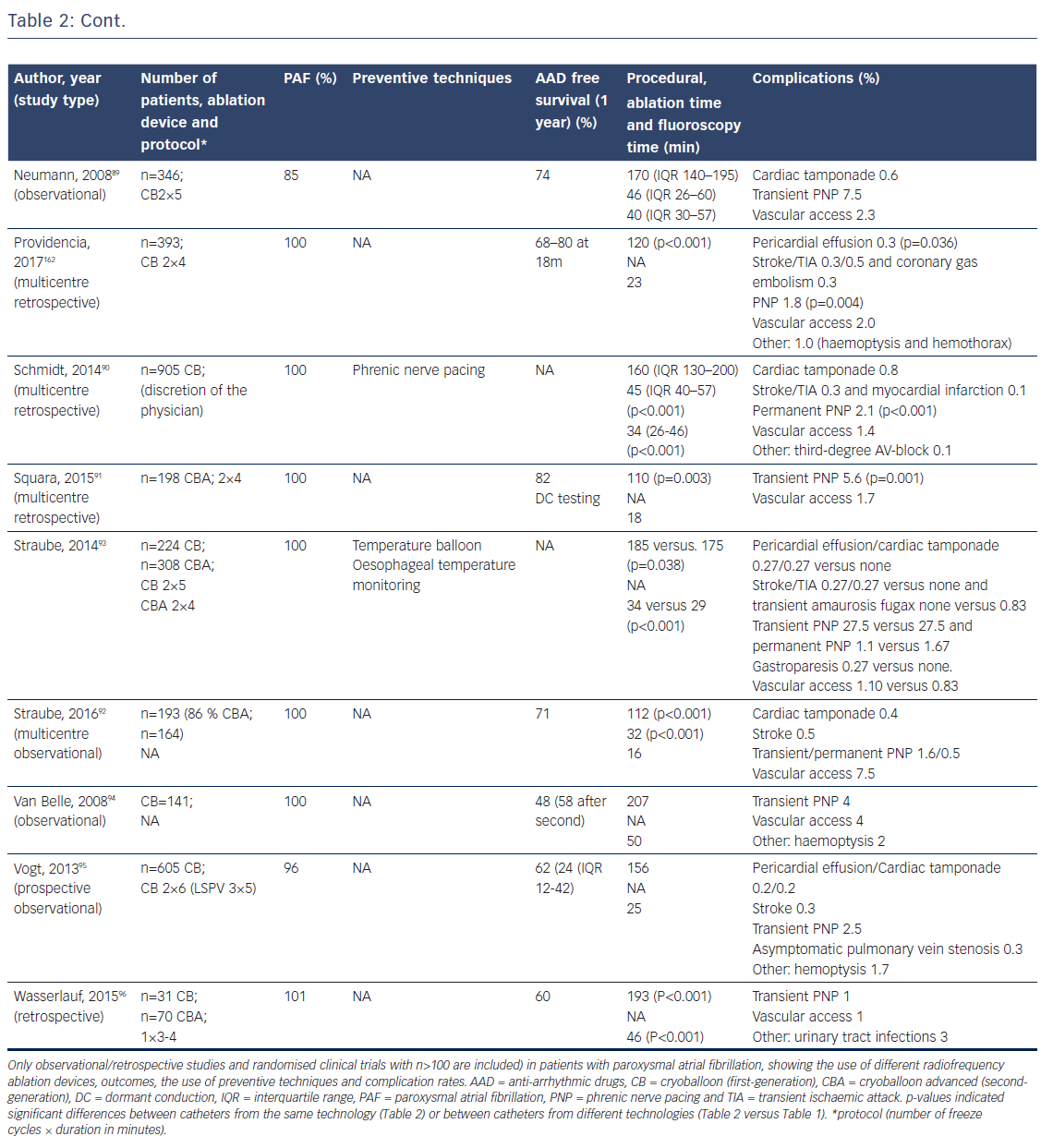
The reported incidence of complications is low and not significantly different between the first and second-generation cryoballoons.79,82–96 Specifically, the reduction in ablation time was not associated with lower complication rates (Table 2). Cardiac tamponade occurred in 0.7 % (47 of 6672 procedures) and was similar for first and second-generation balloons (Table 2). The incidence of phrenic and vagal nerve damage is, however, of concern. In a series of 66 patients, asymptomatic gastroparesis was reported in 9 %, transient PNP in 8 % and symptomatic inappropriate sinus tachycardia in 1 %.97 The reported incidence of PNP ranged between 2 and 28 % for the first-generation and between 1 and 16 % for the second-generation cryoballoon (Table 2). An association between cryoballoon use and any oesophageal injury has been reported in up to 17 %.98,99 However, atrial-oesophageal fistulae are rare and have only been case-reported.100–102 Stroke and transient ischaemic attacks are reported in 0.3–0.5 % of patients (Table 2). Of importance, the risk for PVS is also low. In a recent study, 0.4 % of the patients showed only mild (25–50 %) PVS.103
Hotballoon
Historical Overview
The hotballoon (HotBalloon catheter, Sataka, Toray Industries, Tokyo, Japan) is a compliant RF-based balloon (25–35 mm) that is filled with saline and contrast. The balloon can be heated to a temperature of 65–75°C through a coil electrode inside the balloon. Energy delivery is based on thermal conduction to the tissue in contact with the balloon surface. The first human study has shown that 2–3 applications of 2–3 minutes duration were required to achieve PVI resulting in AF free survival of 92 % off AAD during a mean follow-up of 11±5 months.104 In consecutive studies, reported outcome off AAD was 78, 59 and 65 % after 1, 6.3 and 3.6 years, respectively.105–107 Randomised studies comparing the hotballoon with other ablation technologies are lacking.
Complications
In an early animal study published in 2001, no major complications were reported.108 In a human feasibility study, oesophageal injury, however, occurred in three of the first six cases. After introduction of oesophageal cooling with saline, consisting of repeated injections of 10–20 ml mixture of contrast medium and saline, cooled at 10°C during applications, only one additional injury was observed in the next 58 patients.109 In a series of 502 patients, the incidence of oesophageal injury could be further reduced by adapting the oesophageal temperature cut-off (39°C instead of 41°C).107 Additional procedural-related complications included PNP and PVS. In a series of 319 ablations performed in 238 patients, 16 major complications occurred: >70 % PV stenosis in 4 (1.7 %), temporary PNP in 8 (3.4 %) and oesophageal injury in 4 (1.7 %).105 In a randomised controlled trial comparing hotballoon with AADs, for paroxysmal AF major complications were reported in 15 (11 %) patients: PV stenosis of >70 % in 5 % and transient PNP in 3.7 %.106 The hotballoon is still under investigation and optimal ablation energy and duration needs to be determined.
Laserballoon
Historical Overview
The first-generation laserballoon (Endoscopic ablation system, Cardiofocus Inc. Marlborough, MA, USA) was available in three diameters (20, 25 and 30 mm). It consists of a delivery sheath with an endoscope and arc generator inside a balloon. With the endoscope, the intra-cardiac anatomy and adequate tissue contact can be visualised real-time. The arc generator delivers laser energy to perform PVI.110 Similar to other balloon-based devices, superior caval vein pacing and oesophageal temperature monitoring (39°C cut off) is recommended to minimise the risk for PNP and oesophageal injury. After ablation, PV isolation needs to be evaluated with a separate spiral catheter. In the next-generation balloon (HeartLight, Cardiofocus, Inc., Marlborough, MA, USA), the arc of the laser was decreased from 90–150° to 30° to improve safety. In addition, the balloon material was modified allowing variable sizing and deformation to prevent mismatch between the balloon size and the PV diameter.111 Based on data from nine studies including 1021 patients, the efficacy of the HeartLight balloon procedure ranged between 58 and 88 % at 1–1.5 year follow-up (off AAD).112 A more compliant laserballoon is currently being developed (HeartLight Excalibur Balloon™, Cardiofocus Inc.).
Procedure Time
The first-generation laserballoon was initially constructed as a two-operator device for positioning the balloon and directing the laser ablation.113 The second-generation laserballoon can be used by a single-operator. In addition, energy delivery has been modified leading to a shorter procedural duration from 334 minutes during first use110 to 133–236 minutes in the improved laserballoon.112,114
Complications
A paper providing pooled data of eight small studies (total 308 patients) reported PNP in 2.3 % and cardiac tamponade in 1.9 % of the patients.113 In a multicentre study including 200 patients with paroxysmal AF, similar complication rates were observed (2 % cardiac tamponade and 2.5 % PNP.115 However, in a recent multicentre prospective study 1 patient out of 68 showed PNP and 1 patient developed a stroke (both 1.5 %).114 Of concern, the incidence of asymptomatic cerebral embolism with the laserballoon was 24 %, but not significantly higher (p=0.8) than for the cryoballoon (18 %) and irrigated RFCA (24 %) in a randomised study.116 In a clinical trial comparing laserballoon with irrigated RFCA (178 versus 175 patients), the incidence of all adverse events was also similar (12 % versus 15 %).111 However, the incidence of PNP was significantly higher with the laserballoon (3.5 % versus 0.6 %). PNP was also the major complication in another study with an incidence of 5.8 %. Cardiac tamponade was reported in 3.5 % of patients.117 In these studies PVS was not reported.
Comparison of Ablation Devices
Ablation Technology and Efficacy
Outcome after cryoballoon ablation versus point-by point RFCA has been well studied, also in randomised trials: a recent meta-analysis of 10 studies (total of 6473 patients; 3 randomised trials) showed similar efficacy.118 Data comparing other single-shot techniques with RFCA are limited. Smaller studies suggest no significant differences in efficacy. A randomised multicentre clinical trial comparing the laserballoon with RFCA (178 versus 175 patients) reported a 61 versus 62 % AF free survival at 1 year off AAD.111 Also in another multicentre prospective trial comparing the laserballoon (n=68) with RFCA (n=66) there was no difference in outcome (71 versus 69 %, p=0.40) at 1-year follow-up (off AAD).114 In a study comparing the laserballoon with the cryoballoon (n=140) the efficacy at 1 year off AAD was comparable between the two techniques (73. versus 63 %).119
Ablation Technology and Procedural Time
The reported procedure times for cryoballoon ablation are significantly shorter compared with point-by-point RFCA (Tables 1 and 2).118 Similarly, procedural times using multi-electrode ablation catheters (PVAC) are shorter if compared with point-by-point RFCA, while the efficacy was similar.120,121 Although in an early study longer procedural times were reported for laserballoon ablation compared with cryoballoon ablation and point-by-point RFCA,116 a recent study demonstrated similar procedural duration (laserballoon 144 minutes, cryoballoon 136 minutes).119 This was also applicable when comparing laserballoon with RFCA (128 versus 135 minutes).114
Pericardial Effusion/Cardiac Tamponade
Radiofrequency ablation compared with balloon-based devices is associated with an increased risk for cardiac tamponade (1.5 versus 0.1 %).122 This risk was higher in PVI plus additional lesion sets compared with PVI only (0.8 versus 0.1 %, p=0.024).122 For CF catheters, the reported incidences are higher (2.5–8 %).123–125 Based on published data (Tables 1 and 2), the estimated incidence of pericardial effusion/cardiac tamponade is approximately 1.9 % (144 of 9793; range 1–12 %) for point-by-point RFCA and 0.7 % (47 of 6772; range 0–8 %) for the cryoballoon.
Stroke/TIA
Cryoballoon ablation has been associated with a lower risk for thrombus formation compared with RFCA.126 In line with this data is the observed lower incidence of silent cerebral embolism compared with irrigated RFCA and PVAC.51,52,127 However, in a randomised study comparing laserballoon (n=33), cryoballoon (n=33) and irrigated RFCA (n=33), the incidence of asymptomatic cerebral lesions was not significantly different (24 %, 18 % and 24 %, respectively).116 For PVAC, a higher rate of micro-embolic signals and asymptomatic cerebral embolism has been observed compared with cryoballoon or RFCA.51,53,56 However, the incidence of symptomatic cerebral events (stroke/TIA) is similar (0.3 versus 0.2 %).
Phrenic Nerve Palsy and Oesophageal/Vagal Nerve Injury
The incidence of PNP is significantly higher with the cryoballoon compared with RF, occurring in 3.9 % of the ablations (264 of 6772 cases; range 0–15 %), with permanent paralysis in <1 % (Tables 1 and 2). Similarly, laserballoon ablations are complicated by PNP in 5.8 % of patients.111 In contrast, the reported risk for oesophageal injury is lower with cryoballoon compared with RFCA.128
Pulmonary Vein Stenosis
In a clinical trial comparing laserballoon versus RFCA, the incidence of PV stenosis was lower (0 versus 3 %).111 In a study comparing the laserballoon with RFCA and cryoballoon, only mild stenosis was seen in 18, 10 and 3.6 % of the PVs, respectively.129
Groin Complications and Bleeding
Based on the published data summarised in Tables 1 and 2, there were no significant differences in groin-related complications between cryoballoon ablation and RFCA: total reported cases for cryoballoon are 139 (1.8 %) versus 179 (1.8 %) for RFCA.
Patient Characteristics Related to Complications
The majority of patients included in ablation studies are male.130 Bleeding complications (groin-related) after catheter ablation were reported in 2.1 % of female patients (total 3265 patients, n=518 females) undergoing AF ablation. These numbers exceed those reported in males (n=27; 0.9 %).130 Both female gender and higher age have been associated with major adverse events.29 In a large nationwide survey, significant predictors for complications were female gender, high burden of comorbidity and low ablation volume of the hospital (<50 procedures/per year).131 In addition, patients with diabetes are at risk specifically for thrombotic or haemorrhagic complications.132
Prevention of Complications
Knowledge of all potential complications is important for prevention. Technical advances may help to improve safety. Three-dimensional electro-anatomical mapping and image integration can minimise radiation exposure. Careful procedural planning, close cooperation of different medical specialities (e.g. in hybrid AF treatment) and patient monitoring can further reduce complications.133
Pericardial Effusion/Tamponade
For prevention of cardiac tamponade, limiting of radiofrequency power to 30–40 watts in the anterior wall and 20–30 watts in the posterior wall has been applied in most studies (Table 1a/Table 1b). Previous studies demonstrated that power limitation from 45–60 to ≤42 watts in linear lesions during AF ablation limited the incidence of cardiac tamponade.134 With the introduction of force-sensing catheters, RF power adjustment according to CF parameters became possible. However optimal values remain to be established.135
Stroke/TIA
Trans-oesophageal echocardiography, computed tomography or cardiac magnetic resonance imaging may be used to exclude the presence of a left atrial thrombus.4 Symptomatic cerebral thromboembolic events are relatively rare (0.8 %).136 Independent risk factors are a CHADS2 score ≥2 and a history of stroke.137 Accurate sheath management can reduce the risk of air embolism (incidence <1 %). Continued oral anticoagulation (INR ≥ 2) during the procedure and maintenance of an adequate ACT (>300) should be considered to impact catheter thrombogenicity and the risk for (asymptomatic) cerebral embolism.138 A meta-analysis of 13 studies comparing non-vitamin K antagonists (NOAC) with vitamin-k antagonists (including 3 randomised controlled trials) could demonstrate that NOACs are safe and effective, but adequately-powered randomised controlled trials are required to confirm these results.139
Phrenic Nerve Palsy
Superior caval vein phrenic nerve pacing with palpation ofdiaphragmatic excursions may allow discontinuation of ablation before permanent injury.140 Diaphragmatic compound motor action potential (CMAP) monitoring is a relatively new technique to prevent PNP.141 To measure the CMAP signal, the left and right arm electrocardiogram leads are placed, respectively, 5 cm above the xiphoid and 16 cm along the right costal margin. Peak-to-peak measurement is performed of the CMAP signal with each phrenic nerve capture during superior vena cava pacing with a decapolar catheter. CMAP signals were amplified using a bandpass filter between 0.5 and 100 kHz and recorded on a recording system (Prucka, GE Healthcare, Milwaukee, WI, USA). The technique is well described with figures by Lakhani et al.142 The ablation is terminated after reaching a 30 % reduction in CMAP, which resulted in a faster recovery of phrenic nerve injury compared with manual palpation.143 Abortion of the freeze cycle during cryoballoon ablation (“double stop” technique: immediate ablation termination with direct balloon deflation) is an important additional manoeuvre to prevent permanent nerve injury.143,144 Measuring of CMAP has reduced PNP incidence to 1 % compared to 4–11 % with manual palpation.145
Oesophageal/Vagal Nerve Injury
Reduction of radiofrequency power to 20–25 watts aims to prevent oesophageal injury, atrial-oesophageal fistulae and vagal nerve injury causing gastric hypo-motility.146 Oesophagus and/or vagal nerve damage can be prevented by monitoring of the oesophageal temperature during ablation,147–149 with a reduction from 36 % to 6 % in RFCA150 and from 18.8 % to 3.2 % in cryoballoon ablation.148 Temperature cut-offs that may be considered safe are >38.5°C for RFCA and <15°C for cryoballoon procedures.148,150 However, the use of temperature monitoring during RFCA is still under debate. Employment of temperature probes during RFCA has been associated with a higher incidence of oesophageal injury (30 versus 2.5 %; p<0.01) and using the temperature probe has been identified as an independent predictor.151 It has been hypothesised that the probe may act as an antenna drawing RF energy to the oesophagus.152 Other methods for prevention of oesophageal damage are active cooling with saline,153 changing the oesophagus position with a deviation tool and visualisation of the posterior wall and oesophagus with image-integration and electro-anatomical mapping.154–157 Whether prescription of prophylactic proton-pump inhibitors can prevent oesophageal damage needs further investigation.
Pulmonary Vein Stenosis
Pulmonary vein stenosis is likely an underdiagnosed complication after AF ablation which may be due to the lack of specific symptoms.158 The most important step to reduce the risk of PV stenosis is to avoid ablation inside the PVs by careful determination of the PV ostia before ablation.
Groin Complications and Bleeding
Management of coagulation is important to prevent vascular complications. In addition, a three-point strategy tested in 324 patients with continued warfarin during ablation, a smaller needle for access (18G instead of 21G) and avoiding arterial access has resulted in a reduction in vascular access complications (3.7 % versus 0 %; p=0.03), while the rates of thromboembolic complications and cardiac tamponade were similar.159 Ultrasound-guided versus conventional femoral puncture did not reduce major complication rate (0.6 versus 1.9 %; p=0.62) in 320 patients, however it was associated with significantly lower puncture time, higher rate of first pass success and less extra or arterial punctures.160
Conclusion
Several ablation devices have been developed over the last 15 years to increase procedural efficacy. Improvement of safety profiles is often initiated after the occurrence of complications. Knowledge of potential and device-specific complications and awareness of currently considered asymptomatic procedure related events (e.g. cerebral emboli) is important for patient counselling and selection – primum non nocere.
Clinical Perspective
- Cardiac tamponade remains an important complication and is more frequently observed in irrigated contact-force guided radiofrequency catheter ablation (RFCA) compared with balloon-based techniques.
- Compared with single-shot techniques, the procedural duration of point-by-point RFCA is longer, while fluoroscopy duration is usually shorter due to three-dimensional navigation. High-power short-duration ablation methods are in development to reduce procedural duration with limited data on the safety profile.
- Procedural duration for multi-electrode catheters is short. A potential drawback is the association with asymptomatic cerebral embolism, the clinical significance of which is not clarified yet.
- Improvement of cryoballoon technology has led to shorter procedural and fluoroscopy times with similar efficacy and complication rates. Outcome and complications compared with RFCA are similar, except for a higher incidence of phrenic nerve palsy. Other balloon-based devices are in development with unknown safety profiles.
- Pre-procedural patient evaluation, appropriate device selection, optimisation of energy delivery and intraprocedural monitoring is important to balance efficacy and safety.