Implantable cardioverter defibrillators (ICDs) have been used for over 30 years to prevent sudden cardiac death (SCD). The first indications for ICD placement were secondary prevention; later trials demonstrated a primary prevention benefit of ICD therapy in patients at risk of SCD. ICD therapy prolongs life in both patient populations.1 However, the efficacy of an ICD depends on its ability to correctly detect ventricular arrhythmia and deliver antitachycardia pacing or shocks. In cases of inadequate arrhythmia classification by the device, atrial fibrillation (AF) with rapid conduction to the ventricles can lead to inappropriate shocks. The ability of an ICD to correctly detect this arrhythmia is essential to avoid shock delivery. Furthermore, technical problems such as lead fractures, oversensing of myopotentials or external noise can lead to shock application. ICD shocks can be painful if experienced in the awake state and may reduce quality of life in affected patients.2 Shocks, whether appropriate or not, tend to occur frequently after the implantation of an ICD and avoiding shocks is a reasonable therapeutic target.3
Current evidence suggests that ICD shocks increase the risk of mortality.4 It remains a matter of debate if this finding depends on the context of a specific cardiac substrate or the shock per se.5 Of less controversy is the fact that patients who receive ICD shocks experience reduced quality of life. This has been a consistent finding in published studies that was already evident from data in the first available trials, such as the Antiarrhythmics Versus ICDs (AVID) trial.2 The occurrence of ICD shocks was associated with reduced physical functioning and decreased mental wellbeing. Another study reported that patients who experienced an ICD shock did not adapt well to living with an ICD and were generally more anxious than ICD recipients who received no shock.6 Accordingly, shock reduction by medical, interventional or technical means is a desirable goal.
General Measures
Most patients receiving ICDs for primary prevention of SCD are affected by heart failure with reduced left ventricular ejection fraction (LVEF <35 %). Should these patients meet the criteria for cardiac resynchronisation therapy (CRT), implantation is indicated to achieve mortality and morbidity benefits.7 In addition, CRT responders exhibit fewer ventricular tachycardia/ventricular fibrillation (VT/VF) episodes after implantation than patients not receiving CRT.8 In general, care should be taken to keep these patients on optimal medical therapy including high-dose β-blockers, as these drugs lead to a reduction in rates of SCD and overall mortality in patients with ischaemic and non-ischaemic cardiomyopathy. If such treated patients develop arrhythmias despite β-blocker treatment, an additional anti-arrhythmic treatment will be necessary. In clinical practice, an adjunctive antiarrhythmic is administered to more than half of patients who have an ICD.9
Serum Potassium Levels
Besides pharmacological interventions, simple methods aiming to normalise serum electrolyte levels could be of clinical value. Electrolyte imbalance is an important reversible cause for ventricular arrhythmias and often encountered clinically. In particular, hypokalaemia enhances cardiac electrical vulnerability.10 Conversely, where serum electrolyte levels are normalised, a stabilising effect in terms of arrhythmia incidence has been reported. For instance, a database study including >38,000 patients with acute MI showed the lowest risk of VF, cardiac arrest or death with potassium concentrations of 3.5–4.5 mmol/l.11 In contrast, hyperkalaemia may lead to intermittent or permanent loss of capture or T-wave oversensing. While the former may lead to arrhythmia induction, the latter could lead to double counting and inappropriate shock delivery.12 Accordingly, care should be taken to keep potassium levels within the normal range. While a high-tonormal range may be preferred, this certainly needs to be adjusted to account for the clinical characteristics of individual patients. Understanding the role of electrolyte imbalance for arrhythmia incidence is of particular importance as mineralocorticoid receptorblocker treatment is a guideline-recommended aspect of heart failure therapy.13 Care should be taken if patients receive such treatment as an increase in hyperkalaemia-associated deaths became evident after the publication of the Randomized Aldactone Evaluation Study (RALES).14
Adjunctive Antiarrhythmic Therapy to Reduce Shocks
Although modern ICDs incorporate antitachycardia pacing to treat ventricular arrhythmias prior to shock application, the efficacy of such pacing therapy is sometimes limited. Furthermore, inaccurate classification of non-life-threatening tachyarrhythmia, such as supraventricular tachycardia (particularly AF) as ventricular in origin, increases the rate of inappropriate ICD shocks. These drawbacks associated with the use of ICDs raise questions of how and when to administer adjunctive anti-arrhythmic drug therapy in order to reduce the rate of ICD shocks and improve quality of life.15
Class III Antiarrhythmic Drugs
Vaughan–Williams class III drugs that prolong cardiac action potential duration and thus refractoriness are indicated for suppressing atrial and ventricular arrhythmias. In general, antiarrhythmic drug treatment needs to be weighed against risks and potential side-effects. To date, there has been no study demonstrating a positive effect of antiarrhythmic drug therapy on mortality in patients with an ICD. In addition, in the light of reduced systolic left ventricle (LV) function one has to consider that antiarrhythmic drugs in general further suppress contractility. Only one study has demonstrated an increase in systolic LV function in patients with heart failure treated with amiodarone.16 This finding has not been replicated by other trials.
Amiodarone and Sotalol
The Optimal Pharmacological Therapy in Cardioverter Defibrillator Patients (OPTIC) study represents the largest randomised trial (n=412) comparing the antiarrhythmic drugs amiodarone, d,l-sotalol and β-blockers for ICD shock reduction.17 The OPTIC study included patients with a secondary prevention ICD indication and found that amiodarone in conjunction with a β-blocker greatly reduced the risk of both appropriate (HR 0.30, 95 % CI 0.14–0.68, p<0.05 versus β-blocker alone) and inappropriate (HR 0.22, 95 % CI 0.07–0.64, p<0.05 versus β-blocker alone) shocks. In addition to its direct antiarrhythmic effects on supraventricular and ventricular arrhythmias, amiodarone affects the atrioventricular node by slowing conduction and reducing heart rate, which also may help prevent inappropriate shocks due to supraventricular tachycardia.17 In the same study, d,l-sotalol when compared with β-blockers did not significantly reduce the frequency of ICD shocks; however, a numerical trend towards fewer shocks was evident (HR 0.61, 95 % CI 0.37–1.01, p=0.055 versus β-blocker alone).17
An earlier trial prospectively compared d,l-sotalol with placebo treatment and documented a significant benefit with sotalol in terms of shock reduction.18 Patients were included if they had a secondary prevention indication for an ICD and were stratified according to LVEF function below or above 30 %. In 302 patients with an ICD, d,l-sotalol was titrated up to 160 mg twice daily in addition to concomitant β-blocker therapy. In case of renal dysfunction (30–60 ml/min), one of the daily sotalol doses was skipped. In that trial, d,l-sotalol led to a 48 % relative risk reduction for any shock or death from any cause (HR 0.52, 95 % CI 0.37–0.74, p<0.05) compared with placebo. The incidence of inappropriate shocks for supraventricular arrhythmias was also significantly reduced (relative risk reduction by 64 %, p<0.05).18 The effectiveness of sotalol did not differ significantly in patients with a systolic LV function ≤30 % versus >30 %.
AF with rapidly conducted ventricular activation is a common cause of inappropriate ICD shocks. Current AF guidelines recommend the use of amiodarone for AF relapse prevention in patients with relevant structural heart disease such as heart failure.19 In contrast, sotalol is not recommended for relapse prevention in the setting of heart failure as there is concern about the safety of sotalol in patients with AF.20 While heart failure patients may be particularly prone to sotalol-induced ventricular proarrhythmia, no increased mortality rate was observed in the specific population of patients with an ICD.18 It can be speculated that the ICD may protect patients from potential proarrhythmic events and, accordingly, sotalol could represent a therapeutic alternative in selected patients with an ICD, particularly those with normal renal function. The effectiveness of amiodarone to reduce the risk of inappropriate shocks is already evident even from small studies. In an observational study of 55 patients evaluating the incidence of inappropriate shocks after ICD implantation, amiodarone was most effective in reducing the rate of inappropriate shocks and was superior to β-blockers.21
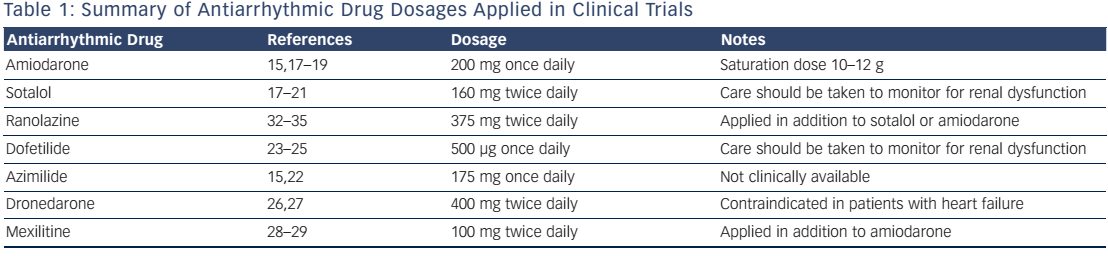
The finding of amiodarone being the most potent antiarrhythmic drug for overall shock reduction is supported by a meta-analysis of 1889 patients in several trials.15 This report pooled data from the abovementioned prospective trials with several retrospective observational studies, and found that the risk of overall ICD shocks was reduced when comparing amiodarone plus β-blocker with β-blocker alone (HR 0.27, 95 % CI 0.14–0.52). Similarly, in that meta-analysis, sotalol was found to be superior to placebo (HR 0.55, 95 % CI 0.40–0.78), but not to other β-blockers (HR 0.61, 95 % CI 0.37–1.00]).15 This metaanalysis was largely supported by the results of the OPTIC study. Given the tendency towards a greater effect of sotalol over β-blockers in the OPTIC study and a statistically significant effect of sotalol in the trial by Pacifico et al., it appears reasonable to keep both substances in mind for shock prevention, realising that amiodarone is most effective (see Figure 1).
Azimilide and Dofetilide
Azimilide is an experimental class III agent that is not clinically available. The meta-analysis by Ferreira-Gonzalez et al. looked at trials of patients treated with class III substances azimilide or dofetilide for shock reduction.15 It failed to document a relevant effect of these two drugs on shock reduction in comparison with placebo (azimilide: HR 0.78, 95 % CI 0.58–1.04, and dofetilide: HR 0.67, 95 % CI 0.43–1.04). In one prospective randomised study involving 633 patients with an ICD, two doses of azimilide were compared with placebo.22 This study documented a dose-dependent suppression of the incidence of ICD shocks by azimilide. However, after addition of data from several smaller dose-ranging studies these effects could no longer be observed.15
In contrast to azimilide, dofetilide is available in several countries. The Danish Investigations of Arrhythmia and Mortality on Dofetilide with Congestive Heart Failure (DIAMOND-CHF) trial suggested a mortality benefit for patients with heart failure with an LVEF <35 % who converted from AF to sinus rhythm on dofetilide.23 The trial was designed to study safety and efficacy of this class III antiarrhythmic drug in patients with heart failure and AF. In that study, ICD placement was an exclusion criterion, thus no data regarding incidence of shocks were available. One recent report suggests effectiveness of dofetilide in reducing the frequency of ventricular tachyarrhythmias in patients with an ICD even after failure of amiodarone. In that observational series 30 patients with symptomatic VT/VF episodes were treated with dofetilide and followed for over 2 years.24 In contrast, oral dofetilide was not effective for shock reduction (HR 0.67, 95 % CI 0.43–1.04, p=NS) in another small study.25 Accordingly, this substance holds some minor potential with an apparent safety profile. It needs to be studied in larger randomised trials.
Dronedarone
Dronedarone was developed to circumvent the untoward side-effect profile of amiodarone, but it is contraindicated in patients with heart failure or permanent AF. The Antiarrhythmic Trial with Dronedarone in Moderate-to-Severe Congestive Heart Failure Evaluating Morbidity Decrease (ANDROMEDA) was initially designed to study a potential beneficial effect of dronedarone on the prognosis of patients with heart failure.26 The trial was stopped early as increased mortality rates were observed with the drug. There are no systematic studies of this substance for ICD shock reduction, but case reports suggest potential benefit in individual patients treated with the substance despite the presence of heart failure.27 The substance should not be used routinely, but may remain a ‘last-resort’ alternative in selected individual cases.
Sodium Channel Blockers
Although the sodium channel blockers are sometimes used as a last-resort therapy in patients with refractory ventricular tachyarrhythmias who fail or do not tolerate amiodarone, there are very limited clinical trial data available.28 In some patients with repetitive ventricular tachyarrhythmias a combination of class I and class III antiarrhythmic drugs – mostly amiodarone – is applied.29 Mexiletine is a Vaughan–Williams class Ib sodium channel blocker. In general, class I drugs should be avoided in patients with heart failure, but in an observational single-centre study, mexiletine given at a dose of 100 mg twice daily in addition to amiodarone 200 mg once daily was effective in reducing the rate of recurrent VT/VF episodes. A combination of mexiletine with sotalol did not have the same effect. However, this effect was only present at short-term follow-up (3 months). At 12 months no such effect remained. This drug may accordingly represent a short-term option for patients with refractory VT/VF episodes.28
Application of mexiletine in patients with long QT syndrome type 3 demonstrates a pathophysiological rationale for use. In this subtype of long QT syndrome a sodium channel gain-of-function mutation underlies QT prolongation and mexiletine shortens the QT interval in this context. It may accordingly reduce ventricular arrhythmias in these patients.30 The class Ic antiarrhythmic drug quinidine has a potential for treatment of arrhythmias in patients with Brugada syndrome.31 These substances may be used to prevent ICD shocks in specific patient populations.
Ranolazine
Ranolazine inhibits the late sodium current that is increased in heart failure and leads to arrhythmia occurence.32 Late sodium current is also a target for AF therapy.33 However, ranolazine displayed only moderate efficacy in a prospective randomised trial of AF relapse prevention.34 In a multicentre observational series of 12 patients, ranolazine was added to sotalol, amiodarone or amiodarone/mexiletine and effectively reduced the rate of ICD shock recurrence.35 Beyond its observational nature, the trial was limited by the small sample size. Prospective randomised trial data are needed for a valid judgement regarding the use of ranolazine as adjunct therapy to reduce ICD shocks. Currently, this substance cannot be generally recommended for ICD shock reduction.
Potential Influences on Pacing and Defibrillation Thresholds
Patients may die from arrhythmogenic causes despite the use of an ICD.36 Reasons for such ICD-unresponsive SCD may be patient or device related, including refractory electrical storm, asystole, pulseless electrical activity or technical defects of the implanted device and leads or potentially elevated defibrillation thresholds (DFTs).37 While there are several reports on changes of DFT by antiarrhythmic drugs in the literature (mostly historical studies and case reports from the 1980s and 1990s), there are few recent data regarding pacing threshold.
Pacing Threshold
Chronic pacing threshold elevation with contemporary steroid-eluting electrode tips is much less of a problem than it was decades ago. However, chronic elevation of pacing thresholds may still occur with longer time since implantation, increasing age of the patient and disease progression.
Antiarrhythmic drugs may alter the excitability of cardiac tissue. While loss of atrial capture has been reported after the application of flecainide and propafenone,38,39 data on ventricular pacing thresholds are highly controversial.40,41 Similarly, loss of atrial capture has been reported with amiodarone,42 whereas no such effect was seen on ventricular pacing thresholds.43 All of these studies were observational in nature. Loss of capture may develop over time in any implantable device. Whether this is truly related to drug administration in these observational patient series is difficult to judge owing to the lack of a control group.
In summary, there is no evidence that any antiarrhythmic drug will consistently alter pacing thresholds in patients. Rather, a specific cardiac substrate may alter pacing thresholds by itself over time.
Defibrillation Threshold
DFT is defined as the lowest amount of energy required to successfully defibrillate the heart and restore normal sinus rhythm. Optimal determination of DFT theoretically includes construction of a continuous dose–response curve between the energy delivered and the success of the defibrillation shock.44
Experimental data and early clinical studies indicate that antiarrhythmic drugs may alter defibrillation energy requirements, usually measured as the DFT.45 Routine ICD testing is no longer recommended as randomised trials evaluating DFT testing versus a non-testing strategy were neutral in terms of predicting effectiveness of ICD therapy.46 The Shockless Implant Evaluation (SIMPLE) study prospectively included patients with a left-sided ICD implant and a no-testing strategy was non-inferior to a testing strategy with respect to the endpoint of failed appropriate shock or death. Data from the Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT) trial indicated no difference in survival if DFT was >20 J in comparison with ≤20 J.47
In a large retrospective analysis, Shukla et al. evaluated data from 968 patients who were enrolled in two separate clinical studies evaluating biphasic shock generators.48 In these uncontrolled studies, 11 % of patients had high DFTs defined as energy requirement ≥18 J. Several indices of advanced structural heart disease, such as NYHA functional class III/IV or low LVEF and the preoperative use of amiodarone, were predictive of higher DFTs.48
The prospective randomised OPTIC trial provided an opportunity to obtain properly controlled data regarding effects of amiodarone, sotalol and β-blockers on DFT with contemporary ICDs. In patients exposed to 6–8 weeks of amiodarone therapy, a minor increase in defibrillation energy requirement of ∼1.3 J was observed, which was statistically significant when compared with a small decrease in DFT in the two other groups. However, a mean 1 J increase in DFT is unlikely to have any effect on outcomes, particularly in light of the potentially high energy delivery with modern ICD systems. Large increases in DFT with amiodarone were rare. In the sole case with a >10 J increase in DFT, an adequate safety margin of 10 J was maintained because of low DFT at baseline (2.5 J). This increase may be due in part to regression to the mean rather than only an effect of amiodarone. In the study, no significant delay in detection of VF on repeat DFT assessment was reported.49 The OPTIC study was the first randomised evaluation to demonstrate that effects of sotalol on DFT are similar to those of conventional β-blockers without class III antiarrhythmic properties.49 Accordingly, it appears generally safe to treat ICD patients with amiodarone and not necessary to perform DFT testing after drug application. Another study suggested that while oral therapy with amiodarone increased DFT during acute ischaemia in a closed-chest animal model, dronedarone in the same model had no effect on DFT either under basal state or after acute myocardial ischaemia.50
While DFT testing should not routinely be performed (neither at implant nor after administration of amiodarone), special situations – for instance if right-sided implants combined with amiodarone therapy or lead replacements – may still warrant such tests.
Summary and Future Direction
Recent trials of ICD programming have shown a significant reduction of ICD shocks. The management of appropriate shocks is still challenging and may be optimised by assessment and treatment of the underlying ventricular arrhythmias and structural correlate in terms of revascularisation or ablation therapy.
For current clinical practice, d,l-sotalol can be considered to reduce ICD shocks (appropriate or inappropriate) in patients without severe renal dysfunction. If sotalol is ineffective, amiodarone has a greater potential to reduce arrhythmia burden and subsequent shock delivery for a larger amount of potential side-effects. One has to keep in mind that both substances may suppress intrinsic sinus rate and accordingly atrial pacing may become necessary. Further clinical trials are needed to assess the long-term benefit of amiodarone to prevent ICD shocks in the context of potential side-effects. Whether other substances, such as ranolazine or dronedarone, will obtain a role in the management of arrhythmias in patients with an ICD is subject to future investigation. Patients with refractory VT/VF episodes despite amiodarone treatment may experience short-term benefit from the addition of mexiletine.
Antiarrhythmic drugs should only be applied after an arrhythmia has occurred (see Table 1). This does not necessarily imply that ICD shocks need to have occurred. All prospective trials looking at shock reduction conducted to date included patients who had already experienced ICD shocks. Whether an a priori preventive therapy with antiarrhythmic agents might be beneficial remains to be studied. Such an approach could represent an additional option to sustain quality of life in ICD patients. In particular, documented AF in a patient with an ICD should be managed with specific programming and potentially antiarrhythmic drug therapy.