The role of cardiac pacing in patients with reflex syncope has been discussed controversially in the last two decades.1–3 Recent multicentre randomised clinical trials, however, have demonstrated the benefit of dual-chamber pacing, leading the European Society of Cardiology (ESC) to provide strong recommendations on patient selection and the diagnostic pathway in their 2021 guidelines on cardiac pacing, to obtain the maximum benefit from pacing in such a benign but disabling condition.4–7 Today, the role of cardiac pacing is finally established and can change medical practice significantly.
There is a growing interest among electrophysiologists in dual-chamber pacing using the closed-loop stimulation (CLS) algorithm.8–10 Although a few small, controlled studies and retrospective analyses suggested a benefit of CLS in reflex syncope compared with conventional dual-chamber pacing, the real effect of CLS on reflex syncope has not yet been fully confirmed, and a large randomised trial is needed to demonstrate any added benefit of CLS over conventional dual-chamber pacing and other syncope algorithms.11,12
The aim of this article is to review the most recent literature, to describe the CLS algorithm, to report plausible evidence-based hypotheses of CLS activation during reflex syncope, and to provide practical advice on the use and programming of the CLS algorithm based on our experience.
Pacing in Reflex Syncope: Which Patients?
Reflex syncope is a very common condition, associated with parasympathetic hyperactivity elicited by central, orthostatic, somatic, and visceral triggers. In most cases clinical presentation is characterised by clear triggers and prodromes. In rarer cases, triggers are uncertain and prodromes are fleeting or completely absent (atypical forms).13 The underlying physiological mechanism basically involves two components: vasodilation and cardioinhibition. Vasodilation generally occurs in all reflex syncope and is complicated by cardioinhibition in some.14,15 Concomitant vasodilation, even in patents with cardioinhibitory forms, still represents a controversial point.
According to the current 2018 ESC guidelines for the diagnosis and management of syncope, the therapeutic approach to reflex syncope should be mainly driven by the patient’s age and the severity of syncope episodes.13 Patients who may benefit most from cardiac pacing are ≥40 years old, have severe clinical presentation of reflex syncope, and show ECG evidence of cardioinhibition during carotid sinus massage, head-up tilt test (HUTT), or implantable cardiac monitoring (ICM). The 2021 ESC guidelines on cardiac pacing have now ranked these conditions as a class I indication for cardiac pacing with level of evidence A.7 The age cut-off of 40 years is related to the inclusion criteria of previous randomised trials. Severe reflex syncope forms are characterised by significant impairment of quality of life, traumas due to syncope episodes, and high-risk professional life because of recurrent or unpredictable episodes with no or very short prodromes.
In this highly selected patient group, cardiac pacing plays an important role in improving quality of life, reducing syncope burden, and/or prolonging prodromes, which may be important for improvement of symptom management. It is worth underlining that pacemaker therapy will not completely eliminate recurrence of syncope in the long term due to a possible accompanying vasodilation.
Evidence from Randomised Clinical Trials
In 2012, the ISSUE-3 trial first showed the efficacy of dual-chamber pacing with the rate drop response function versus no pacing in reducing syncope recurrences in ≥40-year-old patients with severe asystolic reflex syncope documented on ICM (≥3-second syncope or ≥6-second asymptomatic pauses).4 The rate drop response function operates on top of a dual-chamber mode, initiating high-frequency pacing (110–120 BPM) for a programmable time interval, in response to sudden drops in spontaneous heart rate. The absolute and relative syncope risk reductions were 32% and 64%, respectively. Nevertheless, 25% of patients in the pacing group had a recurrence after 2 years.
In 2015, these results were corroborated by the SUP-2 study, an Italian prospective multicentre observational study investigating the role of dual-chamber pacing with the rate drop response feature in patients affected by severe, unpredictable reflex syncope.16 Following a standardised diagnostic algorithm, ≥40-year-old patients who had an asystolic event documented on carotid sinus massage, HUTT or ICM (in this specific order) underwent pacemaker implantation, while the others continued long-term ICM. The 137 patients with a pacemaker had a significantly lower actuarial syncope recurrence rate at 3 years (20%) than 142 patients without pacemaker (43%), regardless of the index diagnostic test. In agreement with previous data, cardiac pacing reduced syncope burden and improved quality of life. However, transient loss of consciousness was not totally abolished due to concomitant vasodilation. Interestingly, in the subgroup of 81 patients who underwent HUTT and eventually received pacemaker based on any index diagnostic test, the probability of syncope recurrence was only 5% after a negative HUTT and 24% after a positive HUTT (asystolic or non-asystolic). A similarly low rate after negative HUTT had also been observed in the ISSUE-3 sub-study.17 These findings shed light on the role of the HUTT in the diagnosis of reflex syncope: if an asystole is documented on long-term heart rate monitoring, a negative response to HUTT is predictive of a pure cardioinhibitory component at the origin of reflex syncope. Conversely, a positive response to HUTT cannot exclude vasodilation, regardless of whether symptoms elicited by the test are associated with an asystole.
Therefore, the last piece of missing information was whether a positive cardioinhibitory response to HUTT is sufficient to select patients who may benefit from cardiac pacing (before starting long-term heart rate monitoring). This was the objective of the recently concluded multicentre randomised placebo-controlled BIOSync CLS trial.6 This patient- and outcome assessor-blind trial verified the efficacy of dual-chamber pacing with CLS in ≥40-year-old patients with ≥2 syncope episodes in the past year, selected based on an asystolic positive response to HUTT. The CLS function was previously tested in reflex syncope with promising results and was therefore selected as the pacing mode in BIOSync CLS.11,12,18 The study had a sequential design with early stopping rules and was based on a rigorous methodology to ensure the blinded adjudication of study endpoints. The 127 enrolled patients underwent pacemaker implantation and were subsequently randomised to dual-chamber pacing mode with CLS (Pacing ON group; n=63) or no pacing with only sensing functions (Pacing OFF group; n=64). The primary endpoint was the time to first post-randomisation syncope. The study was terminated early at the second planned interim analysis due to superiority in the Pacing ON group. The estimated syncope recurrence rate was 19% (Pacing ON) versus 53% (Pacing OFF) at 1 year, and 22% (Pacing ON) versus 68% (Pacing OFF) at 2 years. Dual-chamber pacing with CLS was therefore associated with a 77% reduction in syncope recurrence rate in patients selected for asystolic positive response to HUTT. Of note, almost seven of every ten patients in the Pacing OFF group had at least one recurrence in 2 years. The findings of the BIOSync CLS trial support the inclusion of HUTT as a useful method to select candidates for cardiac pacing. According to a large consortium of experts, accumulated evidence is now sufficient to confirm the central role of the HUTT in the diagnostic route of reflex syncope.19
Mechanisms of Closed-loop Stimulation in Reflex Syncope
The CLS algorithm measures intracardiac impedance curves of the right ventricle during the systolic phase of each cardiac cycle by injecting subthreshold high-frequency unipolar current pulses from the ventricular lead tip.8,9 Given that the density of electric field lines is greatest around the electrode tip, most of the induced voltage drop at each subthreshold pulse and, in turn, the main contribution to impedance, occurs in an approximately 5 cm3 volume (≈1 cm radius) surrounding the electrode, which includes a varying amount of blood and myocardial tissue. Therefore, local changes in the small volume surrounding the electrode (rather than changes in the entire intraventricular volume) are the main drivers of impedance variations. During contraction, blood ejection causes tighter electrode contact with myocardial tissue (involving a larger proportion of tissue relative to blood volume), leading to a progressive impedance increase towards late systole.
Although the operating principle of CLS is sufficiently clear in chronotropic incompetence, the CLS activation during a reflex syncope remains a matter of investigation. Until detailed physiological studies become available, it is possible only to speculate on the results of the studies presented here.
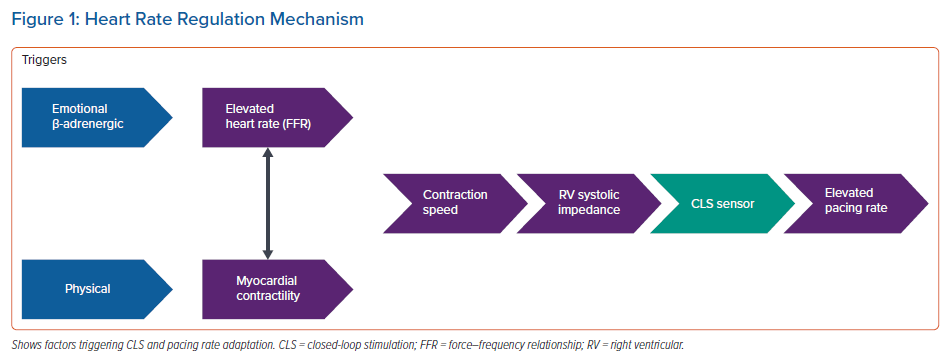
Figure 1 illustrates a possible activation scheme. When reading the scheme from right to left, pacing rate is increased by CLS activation, which is triggered by a detected variation of the shape of rising impedance curves during systole. Changes in right ventricular impedance are strictly correlated to right ventricular dP/dtmax, which is a surrogate of right ventricular contraction speed.10 Contraction speed in turn is influenced by two factors:
- Heart rate: high heart rate causes an intrinsic increase of conduction speed per se, which is reflected by an increase in intracardiac impedance. This is a physiological phenomenon known as positive force–frequency relationship (FFR). Up to 40% of cardiac output is regulated through the positive FFR principle under normal conditions.20 FFR is influenced by β-adrenergic regulation, which operates during exercise, and other conditions affecting the speed of myocardial contraction. In fact, CLS has been shown to react to active standing, handgrip, cold pressor test, mental stress and dobutamine infusion.9,10,21–23
- Myocardial contractility: at given preload and afterload conditions, contraction speed also correlates with myocardial contractility, which is regulated by the Frank–Starling mechanism and by humoral and neural sympathetic innervation. Left ventricular myocardial contractility and sympathetic cardiac tone of the autonomic nervous system indirectly affect contraction speed and intracardiac impedance measured by the CLS algorithm at the right ventricle.
In patients with chronotropic incompetence, cardiac output is mainly regulated via increased myocardial contractility in response to physical triggers (this regulating factor clearly prevails). But it is not completely clear what initiates CLS pacing in patients with impending reflex syncope. We speculate that a heart rate increase and the related positive FFR is dominant in the early (pre-syncope) phase of the reflex (phase 2 of the vasovagal reaction), when the progressive increase in heart rate counteracts vasodilation and pressure drop, starting on average 8 minutes before loss of consciousness, as observed during orthostatic stresses.14,15 Heart rate increases as a consequence of baroreceptor activation and neuroendocrine response to counteract vasodilation.13–15 Contractility is unlikely to come into play at this stage, given that stroke volume has been shown to initially decrease, and the Frank–Starling principle cannot be invoked.14 It is therefore likely that vasodilation ultimately triggers early onset of CLS pacing, as proposed by Sutton et al.3 This hypothesis needs to be confirmed in a future study that would compare stroke volume trends during phase 2 with and without CLS. Also, impedance curves detected by the device would greatly add to the understanding and possible optimisation of CLS.
Closed-loop Stimulation During Tilt-induced Reflex Syncope
The aforementioned speculations are supported by acute HUTT studies. In a cross-over study, CLS was able to increase pacing rate starting from 8 minutes before syncope and to avoid the heart rate drop that precedes syncope by maintaining a constant heart rate at approximately 90 BPM.24 Consequently, the onset of syncope was significantly delayed by 4 minutes and the blood pressure drop was reduced by 22 mmHg. Overall, syncope was induced in 30% of patients in dual-chamber pacing with CLS as compared with 77% in dual-chamber pacing without CLS. The objective is to avoid vagally induced bradycardia. Given that stroke volume and heart rate contribute similarly to the determination of blood pressure at the time of impending syncope, a heart rate increase achieved early through CLS (during phase 2 before syncope) could sustain cardiac output even when the blood pressure begins to fall due to the vasodilation reflex.14
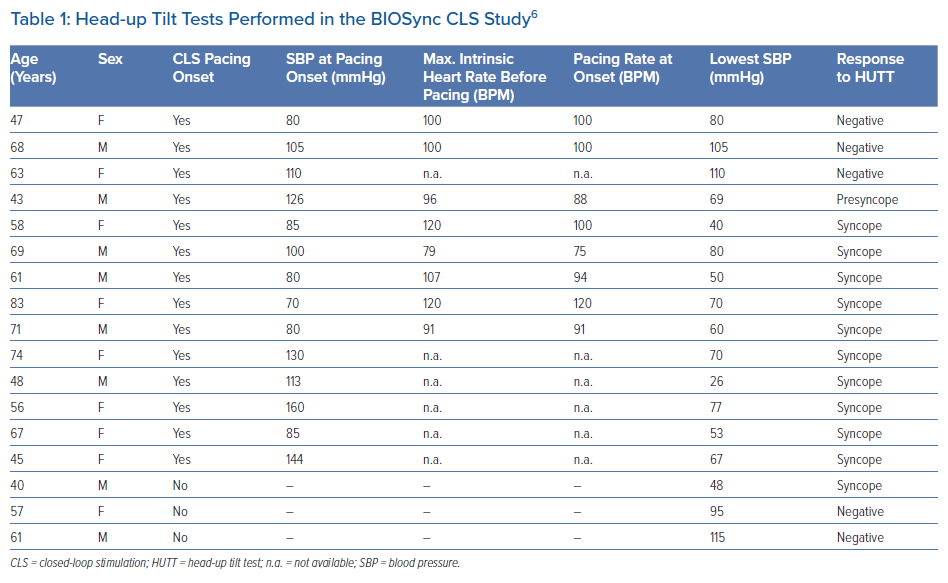
In the BIOSync CLS trial, 17 patients repeated the HUTT after CLS activation (Table 1).6 In 14 of the 17 patients, there was a pacing response. At the time of pacing onset, systolic blood pressure was 105 ± 27 mmHg. Continuous heart rate monitoring data during the test were available in eight patients. At the beginning of phase 2 (cardioinhibitory), the intrinsic heart rate was 102 ± 14 BPM (range, 79–120 BPM) and then decreased progressively. CLS pacing began when the intrinsic heart rate fell to 96 ± 13 BPM. These cases show that CLS pacing is appropriately activated during an orthostatic stress-induced vasovagal response, confirming the study rationale and data from Palmisano et al.24 According to the protocol, the patients remained in an upright position until syncope occurred (n=11) or until the test was completed (n=3). The number of positive responses should not be misleading here, because HUTT is well known to be an unreliable test for demonstrating the effectiveness of pacing therapy in a clinical setting.25,26
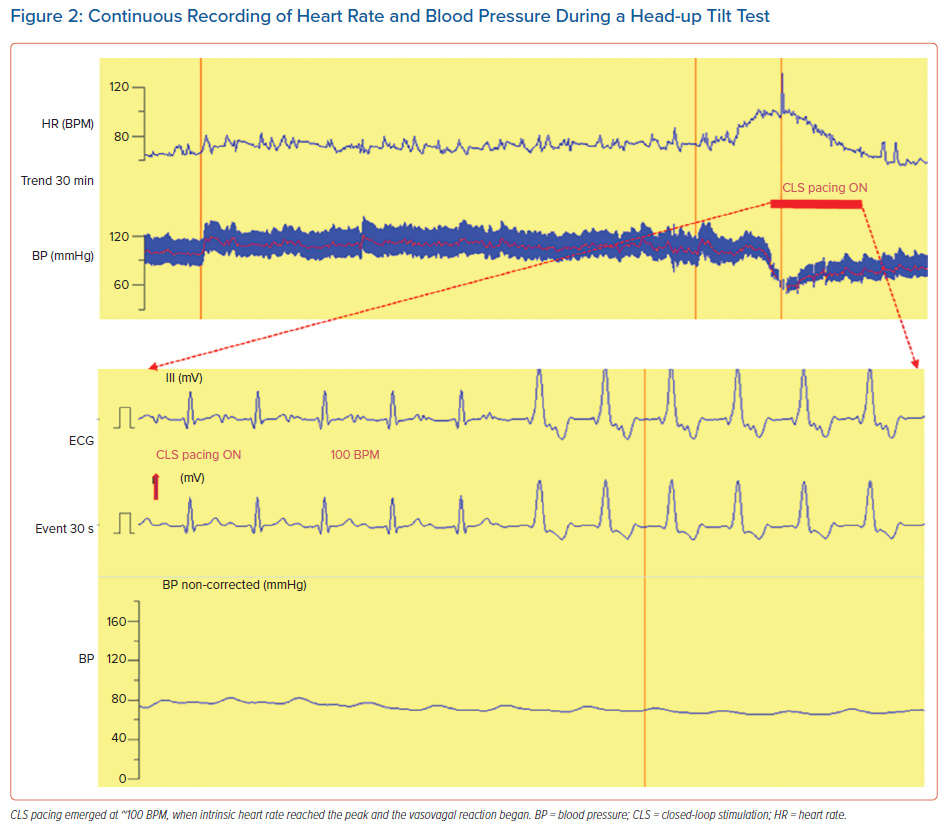
Figure 2 shows a case of activation of CLS pacing in a patient with reflex syncope during HUTT. The heart rate increased spontaneously to sustain cardiac output despite the falling blood pressure (phase 2). At the zenith of the heart rate trend, when blood pressure has approximately reached its nadir, CLS pacing emerged at approximately 100 BPM, first as atrial pacing only and then as sequential atrial and ventricular pacing.
Device Programming and Troubleshooting
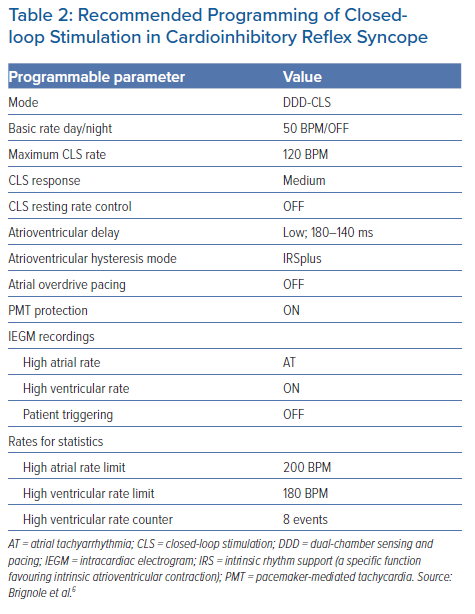
Due to a lack of evidence for what happens in timing and evolution of a syncope attack, it is not possible to make conclusive recommendations for CLS programming. In Table 2 we report the details of the CLS programming used in the BIOSync CLS study, which was shown to be effective in preventing many, but not all, syncope recurrences.6 Therefore, further individual optimisation may be necessary.
Basic and maximum CLS pacing rates are the main parameters that define the maximum range of pacing rate modulation. Basic and maximum rates of 50 and 120 BPM, respectively, should meet most needs, even beyond reflex syncope, although personalisation may occasionally be required. The parameter ‘CLS response’ regulates the amplitude of the CLS reaction to detected changes in the impedance curves: a higher response leads to a faster increase in pacing rate and vice versa.
In the BIOSync CLS study, the setting ‘medium’ did not require any adjustments. The parameter ‘CLS resting rate control’ limits the pacing rate increase when no physical activity is detected by an accelerometer sensor. Due to the special application in reflex syncope and the considerations described here, this limiter was disabled during the study. Although this setting was expected to increase the probability of patient-reported palpitations caused by inappropriately high pacing rates, this adverse event was reported only by one of 63 patients and was resolved by reducing the maximum CLS rate from 120 to 100 BPM. Reducing the CLS response from medium to lower levels would have been an equally valid option to test. Conversely, an increase of the CLS response to a higher level may be worth considering in the case of syncope recurrence.
Finally, the remaining, non-CLS-specific parameters listed in Table 2 are included to describe study settings and may be optimised on an individual basis.
Case Report from the BIOSync CLS Trial
A 48-year-old woman with a smoking habit and no other cardiovascular risk factors was referred to an investigational site for recurrent, unpredictable transient loss of consciousness associated with sweating and dizziness.6 Episodes were not responsive to initial non-pharmacological treatments, including education, lifestyle modification, and psychological counselling. ECG was normal; orthostatic hypotension test and carotid sinus massage were negative. Also, transthoracic echocardiography showed normal ejection fraction and mild mitral regurgitation. HUTT was performed according to the Italian protocol with continuous ECG monitoring and beat-to-beat blood pressure measurements.27 Baseline blood pressure and heart rate were 115/75 mmHg and 80 BPM, respectively. After the 20-minute drug-free passive orthostatic phase, 300 µg sublingual nitroglycerin was given when blood pressure was 125/70 mmHg, and heart rate was 92 BPM. Three minutes later, the patient started complaining of sweating, dizziness, and blurred vision. Blood pressure was 100/60 mmHg and heart rate was 120 BPM. Syncope followed shortly thereafter. A 10-second complete asystole without ventricular escape beats was documented. The HUTT was interrupted, and the patient completely recovered consciousness in a few seconds.
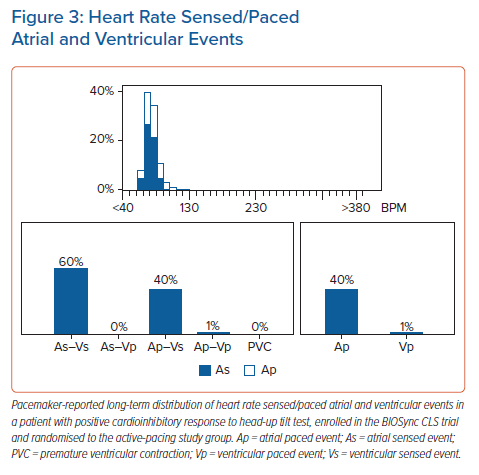
Because of the burden of reported unpredictable syncope episodes and because asystole was the dominant feature of the HUTT-induced syncope, the patient was eligible for enrolment in the BIOSync CLS trial. At 1-year follow-up after pacemaker implantation, the patient did not experience new syncope episodes; the device interrogation showed atrial and ventricular pacing percentages of 40% and 1%, respectively (Figure 3). The atrial pacing rate observed in this case is consistent with the average value in the active group of the BioSync study: comparison of long-term heart rate profiles showed that the CLS algorithm modulated the pacing rates over a wide frequency range, reproducing a heart rate distribution similar in each rate bin to the distribution seen in the control group, despite the average overall pacing rate of 43%.28
Clinical Implications
According to the 2021 ESC guidelines on cardiac pacing, dual-chamber pacemaker therapy is now a class I indication with level of evidence A, in patients with severe, unpredictable, recurrent syncope and an asystole either documented on ICM or induced by carotid sinus massage or HUTT.7 By comparison, in the previous guidelines (2013) cardiac pacing in asystolic HUTT was recommended only with the weakest level of evidence (class IIb).13 The significant increase in the recommendation class was triggered by the latest evidence from the Closed Loop Stimulation for Neuromediated Syncope (SPAIN) and the BIOSync CLS trials.5,6 Both trials compared dual-chamber CLS pacing with sham or disabled pacing. Therefore, the reported benefit of pacemaker therapy is related to the specific pacing mode used in these trials. However, it is not possible to separate the contribution of CLS to the prevention of syncope until the mechanisms of action are clarified and the outcomes are confirmed by a direct comparison of CLS with standard dual-chamber pacing.
Finally, the evidence provided by the BIOSync CLS trial has confirmed the role of the HUTT as an essential step in the decision-making process for pacing in reflex syncope.6
Clinical Perspective
- Recent randomised trials have assessed the benefit of dual-chamber pacing in patients older than 40 years with reflex syncope and asystolic syncope induced by tilt testing.
- Among the available pacing algorithms, the design of closed-loop stimulation is likely to activate pacing early at an adequate rate before a drop in blood pressure, as has been observed during tilt testing. Whether early activation is reproduced in spontaneous clinical episodes is not proven, but it is supported by the positive results from the BIOSync CLS trial.
- Closed-loop stimulation programmability enables a consistent troubleshooting approach. A limited number of patients may still experience syncope recurrence despite pacing. These patients need to be investigated more closely to assess whether relapses should be ascribed to dominant vasodilation or if the pacing mode needs to be optimised.