Introduction
Ventricular tachycardia (VT) is a life-threatening complication of ischaemic heart disease. Sustained VT can be fatal, but is also associated with increased risk of future death and hospitalisation.1
ICDs can terminate VT by delivering therapies in the form of rapid pacing (antitachycardia pacing) and shocks. In conscious patients, these shocks are painful and, unfortunately, they can be delivered inappropriately for non-VT rhythms, resulting in distress for patients. Both appropriate and inappropriate therapies have been demonstrated to worsen outcomes, including mortality.2
Catheter ablation for VT has emerged as a potential treatment to prevent future episodes of VT in patients who have already experienced VT. The ventricles are accessed either via femoral vessels (for endocardial ablation) or the pericardial space (for epicardial ablation).3 Substrate modification, VT mapping approaches or both are then applied to guide ablation with the aim of reducing the occurrence of VT. Guidelines carry only Class II recommendations for VT ablation as an alternative to medical therapy at present.4
Clinical trials of VT ablation are challenging to recruit to and are therefore typically small in population size. Thus, synthesising trial data is particularly important in this field to gain precision to understand the overall effects of VT ablation, especially when assessing prognostic impact. Several recent randomised controlled trials (RCTs) have been published that assess the effects of VT ablation. We therefore conducted a meta-analysis of RCT data, including the most recent trials, to formally evaluate the benefits of VT ablation after MI.
Methods
We conducted a meta-analysis of RCTs that evaluated the effect of VT ablation on all-cause mortality, VT recurrence, ICD shocks and all-cause hospitalisations for patients with VT in the context of prior MI. We conducted the meta-analysis in accordance with the PRISMA statement.5 The protocol was registered a priori on PROSPERO (ID: CRD42023390799).
Search Strategy
We performed a systematic search of the MEDLINE, Cochrane, Embase and ClinicalTrials.gov databases in February 2023 for all studies of VT ablation. Our search strings included ‘ventricular tachycardia’ and ‘ablation’. We also hand searched the bibliographies of relevant selected studies, reviews and meta-analyses to identify further eligible studies.
Abstracts were reviewed for suitability and articles retrieved accordingly. Conference abstracts were eligible where the data for meta-analysis could be obtained. Two independent reviewers performed the search (KS and AN), with disputes resolved by consensus following discussion with a third author (ADA).
Inclusion and Exclusion Criteria
We considered all randomised studies of VT ablation. Studies were eligible if they randomised patients with prior MI, and a prior episode of VT, to VT ablation versus medical therapy and reported outcomes of interest. Observational studies were excluded. Studies were included in this meta-analysis if they either excluded patients without prior MI or if the majority of recruited patients had prior MI.
Endpoints
The primary outcome measure was the HR for all-cause mortality, calculated from extracting digitised Kaplan–Meier curve data. Secondary endpoints were pooled assessment of all-cause mortality, cardiovascular mortality, hospitalisation and recurrence of VT, including appropriate ICD shocks. Subgroup analysis was planned to compare substrate modification approaches with combined substrate modification and activation mapping approaches. Symptomatic and functional data were not assessed because unblinded trials often cannot reliably assess these outcomes.
Data Extraction
Two authors (FS and ADA) independently abstracted trial-level data from included trials with verification by a third author (JS). Digitisation of published Kaplan–Meier plots using open-source software was used to extract time-to-event data, which was then processed to generate individual patient data, as described previously.6,7 The accuracy and reproducibility of this iterative process has been well demonstrated.7–9 This approach allowed individual patient time-to-event data to be analysed, as opposed to pooling of trial-level data in conventional meta-analyses. The accuracy of the reconstructed data was checked by visual inspection and comparison of the published Kaplan–Meier plots to the number and timing of extracted events. Where Kaplan–Meier plots were not available in publications, corresponding authors were contacted via email to request these.
Risk of Bias
Included studies were assessed (by ADA) using the revised Risk of Bias 2 (RoB 2) tool.10 Tests for publication bias were only planned in the event of at least 10 trials being included for analysis.11
Statistical Analysis
We analysed efficacy on an intention-to-treat basis. For the reconstructed individual patient data meta-analysis, mixed-effects Cox proportional hazards regression models were constructed, modelling between-trial heterogeneity as a random intercept to account for differing baseline hazards between the distinct randomised populations.12 The proportional hazards assumption was tested statistically and graphically using scaled Schoenfeld residuals. Kaplan–Meier plots were fitted using the reconstructed individual patient data. We performed trial-level random-effects meta-analyses using the restricted maximum likelihood (REML) estimator. We performed random-effects meta-regression to investigate the relationship between all-cause mortality and the use of substrate modification versus substrate modification in conjunction with VT mapping. HRs and their corresponding 95% CIs were calculated from reconstructed individual patient data. RRs and their associated 95% CI were calculated from event counts for pooled trial-level analysis because not all trials reported HRs for endpoints of interest. We used the I2 statistic to assess heterogeneity.13 Unless stated otherwise, values are expressed as the mean ± SD. The statistical programming environment R (version 4.2.2) was used for all statistical analyses.
Sensitivity Analyses
Prespecified sensitivity analyses were planned to include only trials with 100% of recruited patients having prior MI and to include all trials with any proportion of patients with prior MI. Jackknife analyses with sequential removal of trials were also planned.
Results
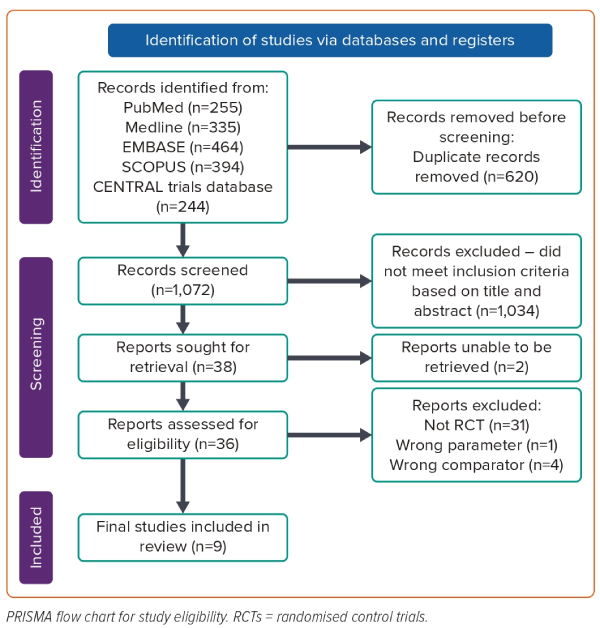
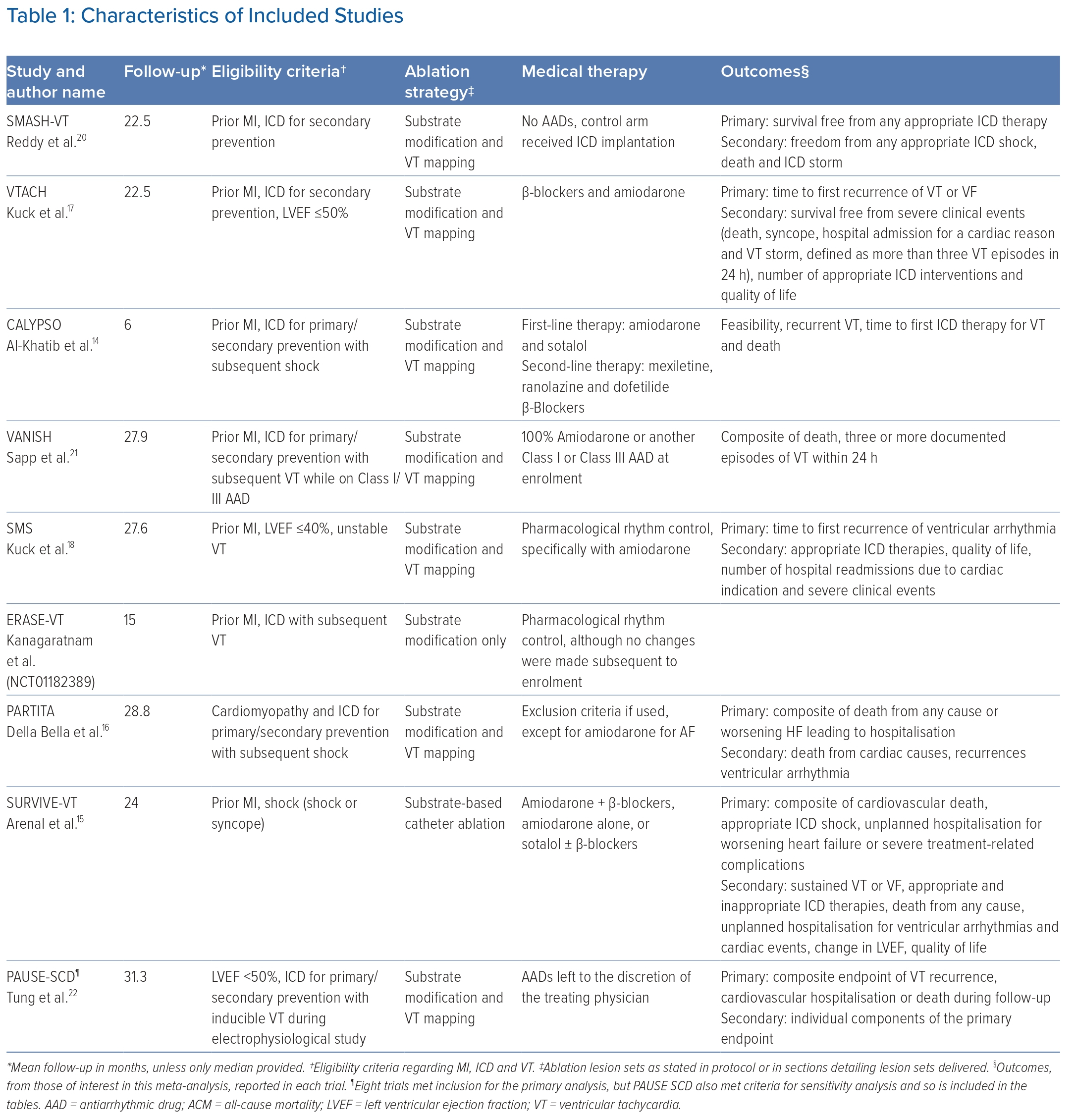
Eight trials, enrolling 874 patients, met the inclusion criteria (Figure 1).14–21 Of these 874 patients, 430 were randomised to ablation and 444 were allocated to medical therapy. Across the eight studies, the mean age of patients was 67.6 years and the mean left ventricular ejection fraction was 31.3%. The mean proportion of patients with prior MI was 97.3%, with six trials recruiting solely postinfarct patients. The characteristics of the included studies and the recruited patients are presented in Table 1 and Supplementary Table 1, respectively.
Trial quality was assessed using Cochrane’s RoB 2 tool, with results presented in Supplementary Table 2. No trial specified blinding of patients, but the trials were generally appropriately conducted in most other respects and were included because the outcomes of interest in this meta-analysis are resistant to bias from lack of blinding. Two trials were graded as intermediate quality due to either insufficient detail or a mid-trial practice change regarding allocation concealment.16,22
Reconstructed Individual Patient Data Meta-analysis
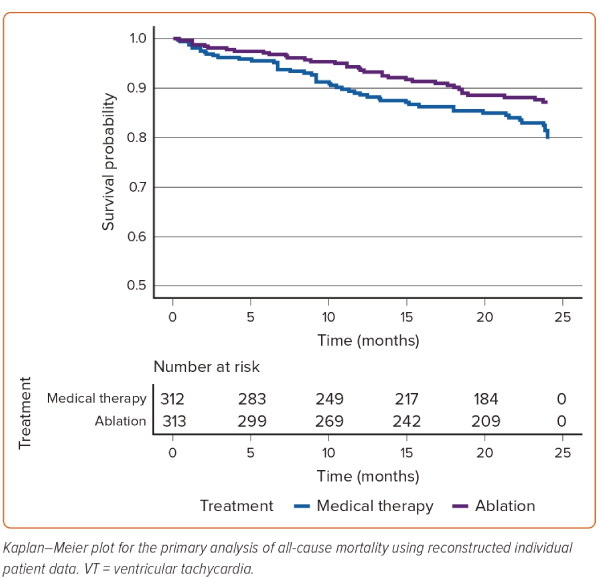
Five trials (SMASH-VT, ERASE-VT, PARTITA, VANISH, SURVIVE-VT) met the inclusion criteria and published Kaplan–Meier curves for mortality, enabling digitisation and subsequent reconstruction of individual patient data (NCT01182389).15,16,20,21 All five eligible trials reported 24-month follow-up for all-cause mortality, and therefore this time point formed the primary analysis. Only one trial reported follow-up to 48 months, so an analysis was also performed at this extended time point.21 Across these five trials, 313 patients were randomised to catheter ablation and 312 were randomised to medical therapy. Catheter ablation resulted in a reduction in all-cause mortality at 24 months (HR 0.63; 95% CI [0.41–0.96]; p=0.03). The 24-month reconstructed individual patient data Kaplan–Meier plot is shown in Figure 2. The proportional hazards assumption was met at 24 months (Schoenfeld p=0.85). Extending follow-up to 48 months, the reduction in the point estimate for mortality reduction remained in favour of VT ablation, but the result was no longer statistically significant (HR 0.73; 95% CI [0.49–1.08]; p=0.12). The 48-month Kaplan–Meier plot is shown in Supplementary Material Figure 1. The proportional hazards assumption was also met at 48 months (Schoenfeld p=0.23). These results remained concordant when the mixed-effects assumptions were relaxed at both 24 and 48 months. There were some discrepancies noted between the absolute event counts tabulated in the reports of two trials (SMASH-VT and VANISH) and time-to-event data obtained by digitisation of Kaplan–Meier curves.20,21 Full details of these discrepancies are presented in Supplementary Material Table 3. To permit comparison with an equivalent trial-level analysis, random-effects meta-analysis was performed including only the trials that published Kaplan–Meier plots, which demonstrated a non-significant reduction in RR favouring ablation (Supplementary Figures 2, 3).
Trial-level Meta-analysis
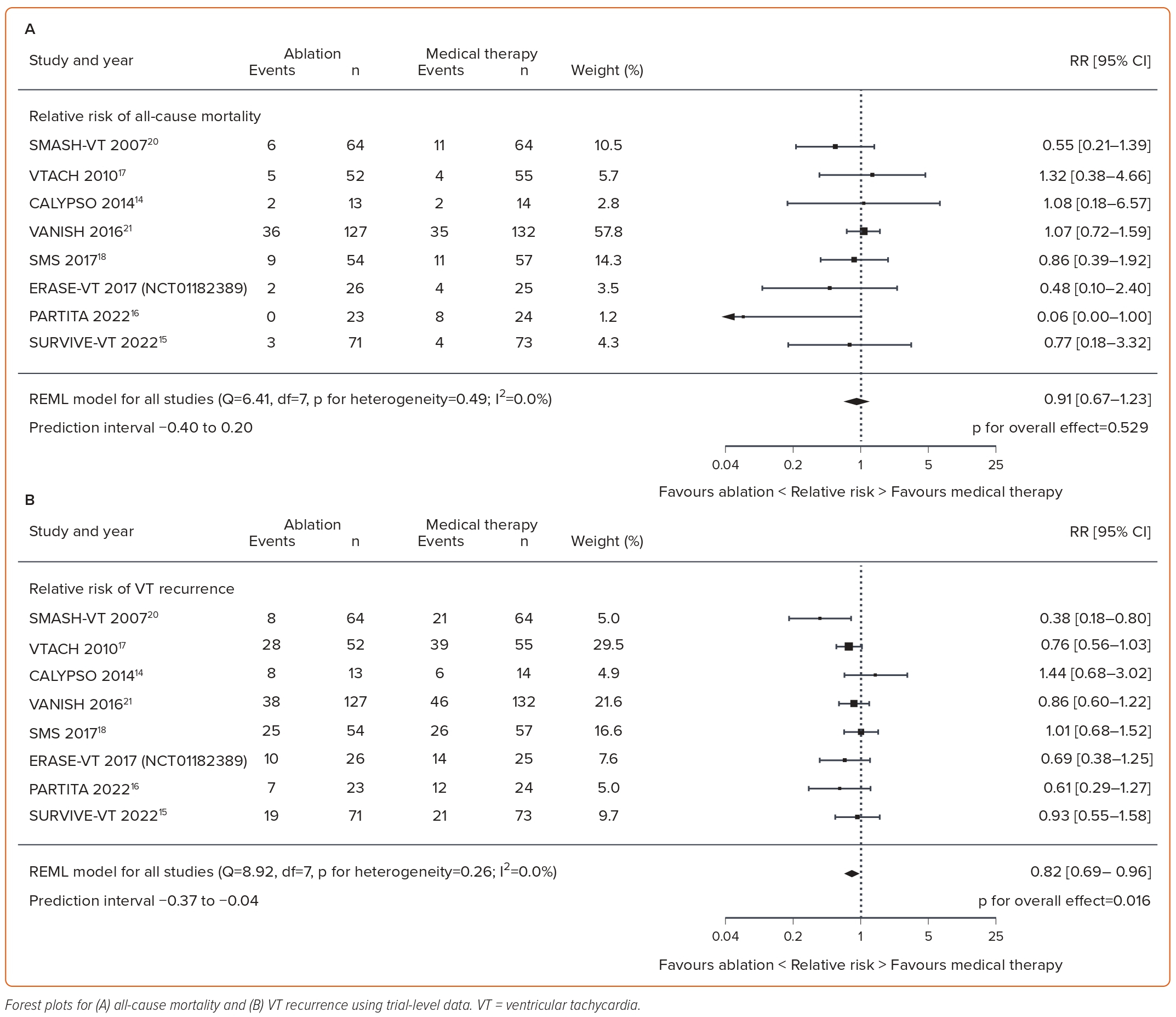
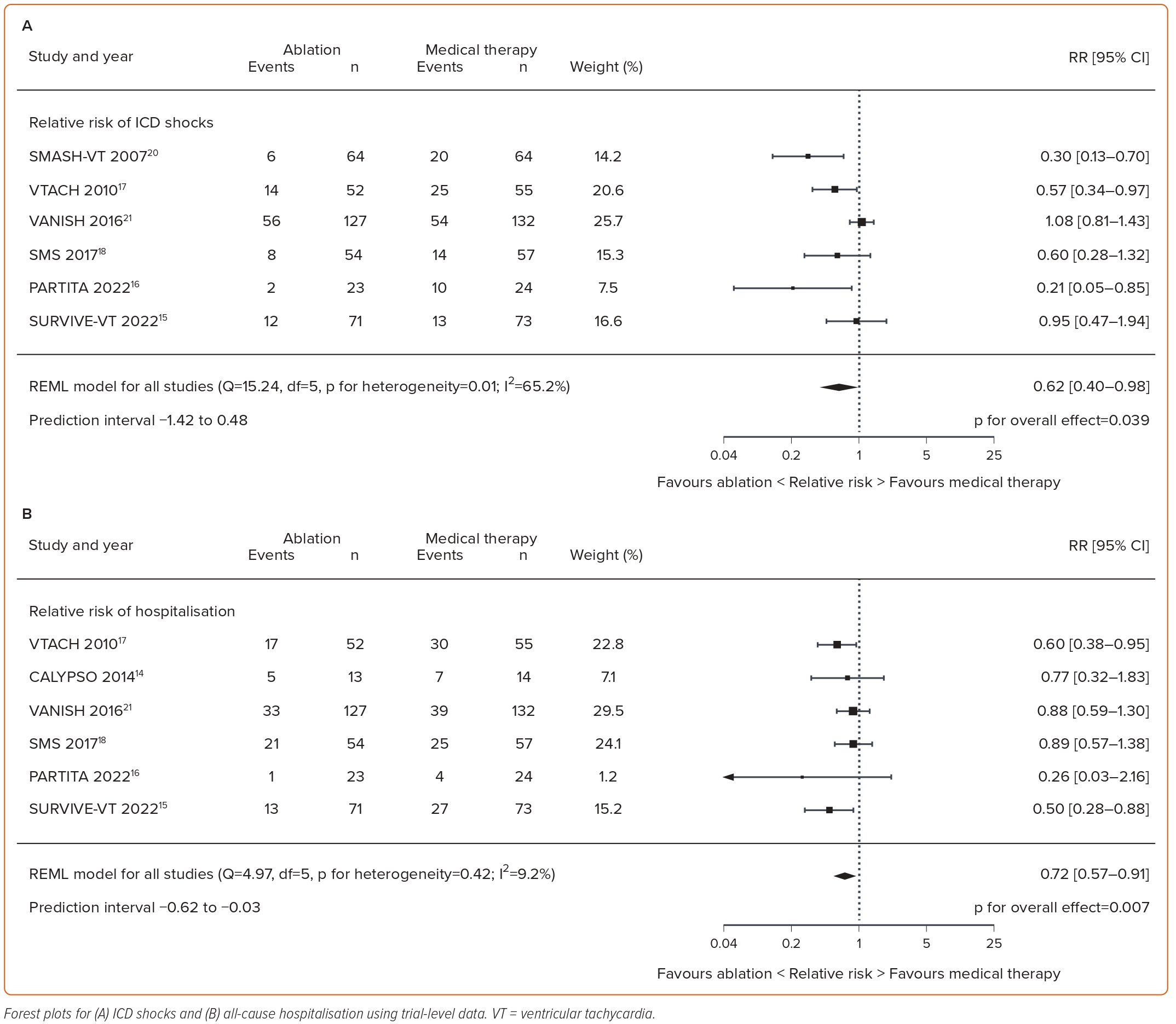
All eight trials that met the inclusion criteria provided data for all-cause mortality.14–21 Catheter ablation did not reduce all-cause mortality in the trial-level analysis (RR 0.91; 95% CI [0.67–1.23]; p=0.53; I2=0%) compared with medical therapy (Figure 3A). However, catheter ablation resulted in statistically significant reductions in VT recurrence (RR 0.82; 95% CI [0.69–0.96]; p=0.016; I2=0%), ICD shocks (RR 0.62; 95% CI [0.40–0.98]; p=0.039; I2=65.2%) and hospitalisations (RR 0.72; 95% CI [0.57–0.91]; p=0.007; I2=9.2%) compared with medical therapy, as shown in Figures 3B and 4A, B, respectively.
Meta-regression
There was no evidence of an interaction of ablation type (substrate modification alone versus substrate modification in conjunction with VT mapping) with all-cause mortality across all included trials (p for interaction=0.16).
Sensitivity Analysis
Jackknife analyses showed that analyses with sequential removal of trials were consistent with the primary analysis (Supplementary Figures 4–7). Prespecified sensitivity analyses were consistent with trial-level analyses. There was no difference in all-cause mortality between catheter ablation and medical therapy in trials recruiting only patients with prior MI (RR 0.95; 95% CI [0.68–1.32]; p=0.76; I2=0%; Supplementary Figure 8). Reductions in VT recurrence, ICD shocks and hospitalisations with catheter ablation also remained consistent with the trial-level analyses (Supplementary Figures 9–11). In trials recruiting patients with any proportion of prior MI, there was no difference in all-cause mortality between catheter ablation and medical therapy (RR 0.92; 95% CI [0.69–1.24]; p=0.60; I2=0%; Supplementary Figure 12). Similarly, the reductions in VT recurrence, ICD shocks and hospitalisations with catheter ablation remained consistent with the trial-level analyses (Supplementary Figures 13–15).
Discussion
In this study, we showed that when individual patient data are analysed, catheter ablation for VT reduces mortality in patients with prior MI. This effect was not seen in the trial-level analysis, which did, however, reveal that ablation prevents VT recurrence, ICD shocks and all-cause hospitalisations. This is the first meta-analysis of VT ablation to analyse individual patient data and is the most comprehensive meta-analysis of VT ablation in patients with prior MI.
European Society of Cardiology guidelines strongly recommend elective VT ablation for the purpose of reducing shock burden in patients with recurrent ICD shocks.4 They provide only a Class IIB recommendation for ablation in patients with ischaemic heart disease and an episode of sustained VT.4 These recently published guidelines specifically state that there is a lack of evidence from prospective, randomised trials that catheter ablation reduces mortality. Our study demonstrates that, in fact, VT ablation does reduce mortality in patients with VT and ischaemic heart disease, but this is only evident when individual patient data are analysed. Prior trial-level meta-analyses have not been able to reveal a mortality effect of VT ablation and our trial-level analysis is consistent with those results.23 By analysing individual patient events extracted from Kaplan–Meier curves, we use time-to-event data to demonstrate a more precise and robust measure of the effect of VT ablation on mortality.
This finding is important for several reasons. VT ablation is an invasive procedure, which carries a risk of tamponade, valve injury, atrioventricular block, stroke, MI, vascular injury and death. Although controlling VT and ICD shocks are valuable goals in themselves, patients may be deterred from the upfront risks of the procedure if there is no hope of improvement in their overall prognosis. The apparent absence of prognostic benefit has led to VT ablation being deployed as a palliative measure after prolonged attempts at medical therapy, which often results in multiple VT recurrences prior to ablation.24 Our finding of mortality benefit repudiates this strategy and suggests that VT ablation can be deployed further upstream in the disease course of patients with VT and ischaemic heart disease.
Our findings of reduced VT recurrence, ICD shocks and hospitalisations with VT ablation are in line with previous meta-analyses.23 This demonstrates that VT ablation is effective in its primary goal, which is to necrose the regions of the myocardium that facilitate VT re-entry circuits. The strategies used to achieve this goal varied between source trials. Three trials (ERASE-VT, SMASH-VT and SURVIVE-VT) used substrate modification alone to achieve this (NCT01182389).15,20 This is where 3D mapping systems are used to identify channels of living myocardium, within areas of scar, that have the potential to support VT circuits. The regions thought to be capable of supporting VT are then ablated. The remaining five trials used VT mapping alongside substrate modification.14,16–18,21 VT mapping involves inducing VT and then mapping the ventricles to identify the precise regions involved in that VT’s circuit. Both techniques have relative advantages and drawbacks. Induced VT may be poorly tolerated and contribute to procedural morbidity and mortality. Furthermore, it can be difficult to determine whether induced VT is the same as clinical VT experienced by the patient, particularly when VT recordings are only captured on ICD electrograms and not 12-lead ECG. When ablating substrate without inducing VT, it is often necessary to ablate large regions of late potentials, even if only a few of those regions are critical to VT circuits. Thus, both mapping of induced (potentially non-clinical) VT and substrate ablation may result in unnecessary ablation of myocardium that cannot support clinical VT. The VISTA trial randomised patients to either substrate ablation or mapped VT ablation.25 Substrate ablation was more effective at preventing VT recurrence and produced a statistically non-significant but numerically lower mortality at the 12-month follow-up (8.6% versus 15%; p=0.21).25 Further research is required to determine the optimal strategy, which may vary from patient to patient.
A key strength of the present analysis was extraction of reconstructed individual patient data from published Kaplan–Meier curves, which allowed generation of HRs, the most appropriate method of summarising time-to-event data. Hazard is conceptually similar to risk but subtly different in that it measures instantaneous risk and may change continuously.26 Reporting an RR for time-to-event data assumes the risk remains proportional over time, which may lead to erroneous conclusions if this assumption is not tested empirically, potentially undermining the validity of a meta-analysis. In addition, by using RRs, information regarding censoring and time-to-events is ignored, draining statistical power from analyses. This may explain why the HR for mortality in the reconstructed individual patient data at meta-analysis reached statistical significance in favour of VT ablation, whereas the trial-level estimate for RR favoured ablation but had a CI crossing unity. There are logistical, resource and privacy constraints surrounding obtaining detailed individual patient data from trial databases. Collaboration between study investigators requires considerable co-ordination. The reconstructed individual patient data meta-analysis approach is therefore a good surrogate and should be considered for survival analysis where Kaplan–Meier curves are available for relevant outcomes.
PAUSE-SCD recruited mostly patients with non-ischaemic cardiomyopathy and, as such, was only included in our meta-analysis in sensitivity analyses.22 This trial also demonstrated the least beneficial trial result for the effect of VT ablation on mortality, with the point estimate favouring medical therapy. Although there are other differences between PAUSE-SCD and the trials that recruited almost exclusively patients with prior MI, there are at least two possible mechanistic explanations for this difference in result being related to the proportion of non-ischaemic patients recruited. After MI, once the VT-supporting scar border zone regions have been ablated, repeated infarcts are required to create new VT-supporting regions. Through revascularisation and medical therapy, the upstream source of these infarcts, coronary artery occlusions or stenoses, can often be successfully prevented. However, non-ischemic cardiomyopathy as a category represents a diverse group of different disease processes, including dilated cardiomyopathies and arrhythmogenic cardiomyopathies caused by various mutations, expressing themselves in varying phenotypes. Once a proclivity to ventricular arrhythmia is recognised in patients with non-ischaemic cardiomyopathy, there are no treatments available that target the causative process. Even once VT-supporting regions are ablated, further regions will emerge.
This explains why VT ablation after MI is more successful at preventing VT recurrence than ablation in patients with non-ischaemic cardiomyopathy, but the lack of mortality improvement in non-ischaemic patients is out of keeping with the relatively minor reduction in ablation success. From this we can hypothesise that VT episodes modify the disease course and prognosis of ischaemic cardiomyopathy to a substantially greater extent than the course of non-ischaemic cardiomyopathy. Therefore, prevention of VT episodes, through ablation, improves prognosis after MI much more than in non-ischaemic conditions. This may arise from the fast heart rate, dyssynchronous activation and abnormal ventricular filling of VT producing demand ischaemia driving worsening of left ventricle function and further substrate for VT. In non-ischaemic cardiomyopathy, VT episodes may be markers, rather than drivers, of disease progression.
The magnitude of benefit from ablation in the included trials was large. All-cause mortality risk was reduced by 37%. Given the high risk of mortality in the medical therapy arms of these trials and in real-world patients, the absolute benefit is likely to be high. This meta-analysis adds to a growing body of evidence suggesting that catheter ablation is an important tool for improving prognosis in heart failure.27
Limitations
We could only report the available data and cannot account for all unpublished trials. Three of the eight eligible trials did not publish Kaplan–Meier curves for all-cause mortality or cardiovascular mortality, and these data were not provided on request.14,17,18 SURVIVE-VT did not publish a Kaplan–Meier curve for all-cause mortality, but did for cardiovascular mortality.15 There was only a one-event difference between these two endpoints (one additional all-cause mortality event in the medical therapy arm). By using the cardiovascular mortality curve, loss of this extra event would favour medical therapy, providing a more conservative estimate of any benefit gained with VT ablation, so this trial was deemed suitable for inclusion in the primary analysis. The sensitivity analysis of conventional meta-analysis including and excluding the three trials without Kaplan–Meier curves did not show a significant difference in result (Figure 4; Supplementary Material Figures 2, 3). Of the 142 mortality events in the eight eligible trials, 109 (77%) occurred in trials publishing Kaplan–Meier curves, and in the remaining trials there were 16 deaths in the intervention arms and 17 in the medical therapy arms, suggesting a low fragility of our result to missing data. Although the trials were of generally low risk of bias, there is a potential risk of residual confounding due to lack of clarity around allocation concealment in some trials. It is not clear whether this would increase or decrease the effect size.
ERASE-VT has not yet published, but we were able to obtain individual patient data and methods for this study (NCT01182389). The method for robotic VT ablation used in this study has been published.19,28 BERLIN VT was a trial explicitly comparing preventive ablation to deferred ablation rather than medical therapy.29 Thus, both arms were randomised to receive VT ablation and as such this trial was not eligible to be included in the present meta-analysis. It should be noted that this trial showed a non-significant point estimate for all-cause mortality in favour of deferred rather than early ablation (HR 2.97; 95% CI [0.60–14.7]; p=0.18).29 ICD use and programming varied between trials. This is reflective of real-world practice where the use, relative timing, programming and subsequent reprogramming of ICD implantation with regard to VT ablation may vary. Between and within the trials analysed in this meta-analysis, there was substantial variation in the tools and specific technical methods for performing VT ablation. This includes different strategies and mapping techniques and different ablation and mapping equipment, and not all ablations were performed in high-volume centres. These effects could not be untangled in this analysis, but future research could be directed towards understanding the optimal approaches.
Conclusion
Catheter ablation for ventricular tachycardia reduces mortality in patients with prior MI.
Click here to view Supplementary Material
Clinical Perspective
- The prognostic impact of VT catheter ablation following MI remains an important outstanding research question.
- In this meta-analysis of eight trials recruiting 874 patients, the effect of VT ablation on all-cause mortality, VT recurrence, defibrillator shocks and all-cause hospitalisations was tested.
- In analyses using reconstructed individual patient data obtained through digitisation of Kaplan–Meier curves, VT ablation was found to reduce all-cause mortality by 37% at 24 months, although study-level meta-analysis, although numerically consistent, was not statistically significant.
- VT ablation also reduced VT recurrence, defibrillator shocks and all-cause hospitalisations.
- This meta-analysis highlights that the synthesis of time-to-event data in meta-analysis rather than simple event counts can provide a more precise point estimate of the effect of an intervention.