Conduction system pacing (CSP) is a therapy that involves the placement of permanent pacing leads along different sites of the cardiac conduction system with the intent of overcoming sites of atrioventricular (AV) conduction disease and delay, thereby providing a pacing solution that results in more synchronised biventricular activation. Lead placement for CSP can be targeted either at the bundle of His, known as His bundle pacing (HBP), or at the region of the left bundle branch (LBB), known as LBB pacing (LBBP). HBP was first described by Deshmukh et al. in 2000.1
There are a number of observational studies that have demonstrated the clinical benefits of HBP over conventional right ventricular (RV) pacing.2–5 LBBP was first described by Huang et al. in 2018 and involves placement of a pacing lead through the inter-ventricular septum closer to the main trunk of the LBB, bypassing areas of AV conduction disease.6 Over the past decade, these techniques have gained significant popularity and specific tools have been designed to enhance lead delivery.
Cardiac resynchronisation therapy (CRT), which has traditionally been performed using biventricular pacing (BVP), in addition to guideline directed medical therapy, is the cornerstone treatment for patients with cardiomyopathy, heart failure (HF) and ventricular dyssynchrony.7 Although not a new concept, HBP and LBBP have been successful in overcoming bundle branch block (BBB) and result in ventricular synchrony, particularly in patients with more proximal disease. This has allowed the use of these strategies for CRT, either as a first-line therapy or as a rescue strategy when BVP fails.
In this paper, we provide a comprehensive review of CSP for CRT including a review of the available data on CSP among various indications for CRT.
Traditional Biventricular CRT
BVP is the conventional form of CRT (BVP-CRT). It is as an integral part of therapy for patients with HF with depressed left ventricular ejection fraction (LVEF) and a wide QRS, which implies inter-ventricular dyssynchrony. Several large, randomised studies have demonstrated improved quality of life, increased exercise capacity, reduced HF hospitalisation and decreased all-cause mortality with the use of traditional BVP-CRT.7–12 The patients who derive the most benefit from BVP-CRT are those with HF with reduced ejection fraction (HFrEF) and left bundle branch block (LBBB). BVP-CRT may also benefit patients who develop an RV pacing-induced cardiomyopathy (PICM), which is another form of ventricular dyssynchrony, and patients with a low LVEF undergoing implantation of a new or replacement pacemaker or ICD with an anticipated requirement for a significant percentage (>40%) of ventricular pacing.13,14
However, about 30% of patients receiving BVP-CRT do not derive a detectable clinical or echocardiographic benefit and some worsen after resynchronisation.7,9,15 Anatomical limitations such as lack of suitable venous branches and unavoidable phrenic nerve stimulation at suitable anatomic LV lead positions affect the success of coronary sinus lead implantation. Finally, certain subsets of patients, such as patients with HFrEF and RBBB or patients with a narrow QRS duration (QRSd) and need for ventricular pacing, may not derive a significant benefit and hence may not be ideal for traditional BVP-CRT.16
Conduction System Pacing for CRT
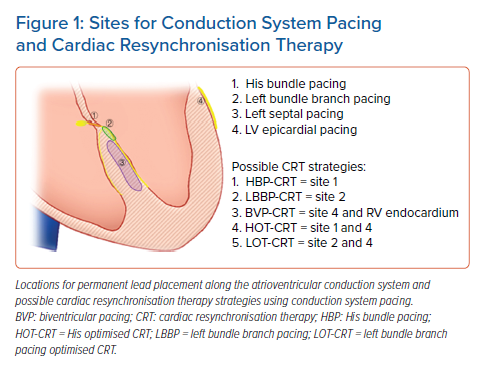
Permanent HBP was first described by Deshmukh et al. for maintenance of inter-ventricular synchrony in a small series of patients with AF and cardiomyopathy undergoing AV node (AVN) ablation.1 Over the past decade, there has been a reinvigoration in the interest in HBP as more data are now available on the benefit of using HBP for patients who need ventricular pacing.2,3,17 However, given challenges with an increase in HBP lead capture thresholds and oversensing in some patients, Huang et al. first described placing a permanent pacing lead more distally along the conduction system in a patient with LBBB and HFrEF with a low capture threshold and this improved outcomes.6,17 This newer location of lead implantation along the LBB region of the conduction system has gained popularity over the past 2 years with growing data on this implant location having low left fascicular capture thresholds, better R wave sensing and potential ease of implantation (Figure 1).
Over the past few years, these two sites for pacing along the cardiac conduction system have become attractive as potential alternatives to BVP-CRT with the demonstration of resynchronised ventricular activation in various studies.18–27 CSP has been used as a primary strategy when CSP is attempted as the first-line therapy for CRT or as a rescue strategy in cases where coronary venous anatomy limits the ability to successfully place an LV epicardial lead.
Data on Conduction System Pacing for CRT
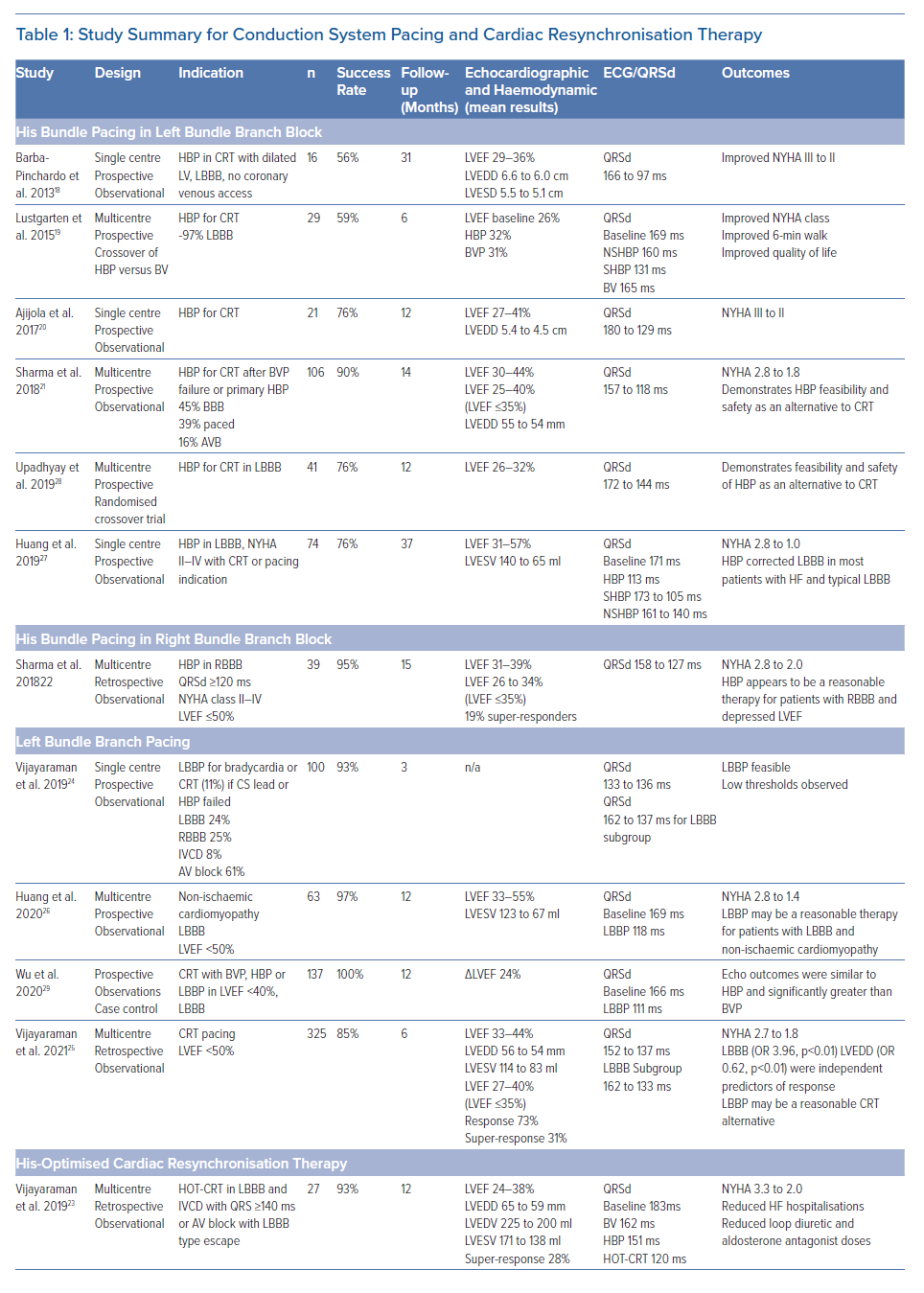
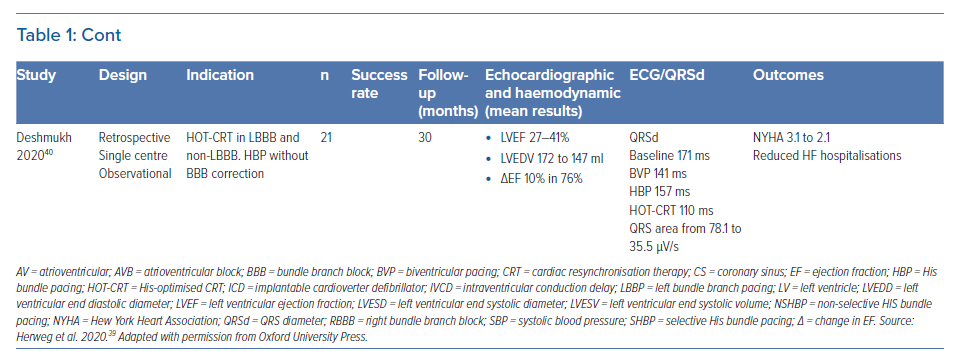
Multiple studies have been published on the benefit of HBP and LBBP as a CRT strategy. However, it is important to recognise that the majority of these studies are observational and non-randomised, with only two pilot studies randomising HBP compared with BVP with a limited number of patients (Table 1 ). Below we review the available data on CSP based on indication for CRT. Figure 1 highlights some pacing locations with possible strategies for CRT.
Patients with Left Bundle Branch Block and HFrEF
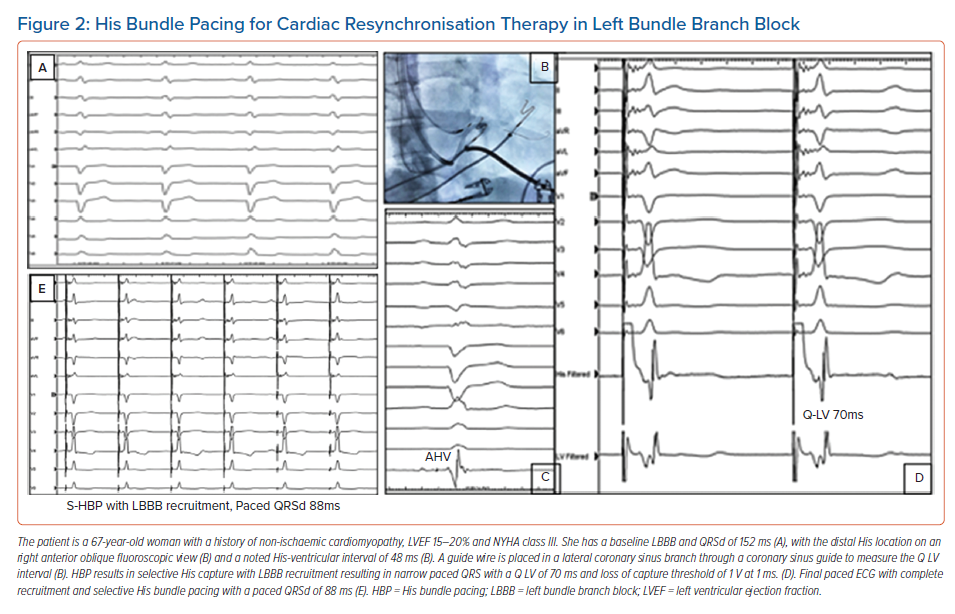
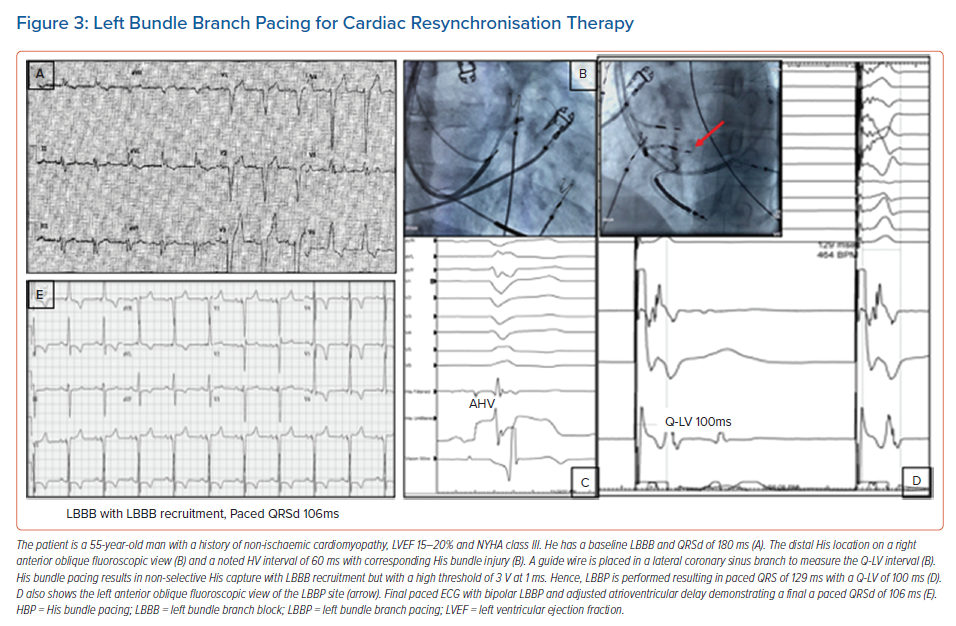
It is well recognised that the patients who derive the most benefit from traditional BVP-CRT are those with LBBB. CSP with HBP and LBBP can recruit and narrow typical LBBB and provide an ideal resynchronisation option. Case examples of HBP and LBBP are demonstrated in Figures 2 and 3. Most of the available data on CSP for CRT focuses on this patient population and this is summarised in Table 1.
Barba-Pichardo et al. first described their experience with HBP in failed CRT cases in 2013.18 HBP was attempted in 16 patients with cardiomyopathy and failed BVP-CRT. Temporary pacing at the HB corrected LBBB in 13 patients (81%). Successful permanent HBP for CRT was achieved in 69% of the patients (n=9). Mean QRSd decreased from 166 ± 8 ms to 97 ± 9 ms. New York Heart Association (NYHA) functional class improved from III to II and there was an improvement in LVEF and LV dimensions. This was one of the first series demonstrating the value of HBP for CRT but the study was limited by its observational nature, small number of participants and low success rates.
Lustgarten et al. performed a crossover study comparing HBP versus BVP-CRT in 29 patients, with successful resynchronisation in 21 (72%) cases.19 All patients received a coronary sinus LV lead and an HBP lead connected to the LV port with a Y adapter and were randomised in single patient-blinded fashion to either HBP or BVP pacing. Among the 12 patients who completed the crossover analysis, patients from both groups demonstrated significant improvements in LVEF, NYHA functional status and 6-minute walk distance. Although this was the first randomised crossover study evaluating the value of HBP for CRT, only 12 of the 29 patients completed the study.
Ajijola et al. evaluated the value of HBP as an alternative approach to CRT in lieu of coronary sinus lead and this was successful in 16 of the 21 patients in the study (76%).20 There was a significant narrowing of the QRSd from 180 ± 23 ms to 129 ± 13 ms (p<0.0001) with an improvement in NYHA functional class from III to II (p<0.001), while the mean LVEF and LV internal dimension in diastole at a median follow-up of 12 months improved from 27 ± 10% to 41 ± 13% (p<0.001) and from 5.4 ± 0.4 cm to 4.5 ± 0.3 cm (p<0.001), respectively. This study was a small observational single-centre evaluation and only focused on patients with LBBB.
Sharma et al. reported a multi-centre study of HBP for CRT in 106 patients including HBP as a primary or rescue strategy and reported an overall success rate of 90%.21 BBB was present in 45% of the patients while 39% cases had a paced rhythm. During a mean follow-up of 14 months, both groups demonstrated significant narrowing of QRS from 157 ±33 ms to 117 ± 18 ms (p=0.0001), increase in LVEF from 30 ± 10% to 43 ± 13% (p=0.0001) and improvement in NYHA class with HBP. His capture and BBB correction thresholds were 1.4 ± 0.9 V and 2.0 ± 1.2 V at 1 ms, respectively. Lead-related complications occurred in seven patients. Although this study was multicentre and included patients with all indications for CRT, the retrospective nature of this study was a limitation.
Upadhyay et al. conducted a multicentre randomised pilot study evaluating the value of HBP for CRT compared with BVP-CRT in 41 patients with CRT indications.28 Of the 41 patients in the study, 35 had an underlying LBBB. Although the crossover rate was high, QRSd was significantly shorter in those that received His-CRT compared to those that received BVP-CRT (125 ± 22 ms versus 164 ± 25 ms; p<0.001). The median change in LVEF was higher for His-CRT compared to BVP-CRT, but this difference was not statistically significant (+7.2% [5.0–16.9%] versus +5.9% [1.5–11.3%], p=0.17). A trend toward higher rates of echocardiographic response (80% versus 57%, p=0.14) was similarly observed. This was the first multicentre randomised pilot study evaluating His-CRT versus BVP-CRT. Some of the conclusions were limited due to the high crossover rates and the inclusion of patients with intraventricular conduction delay (IVCD) QRS patterns that do not always respond to HBP for CRT.
Huang et al. published data on HBP for CRT in 74 patients with non-ischaemic cardiomyopathy and LBBB.27 Permanent HBP was successful in 75.7% of the patients (n=56). Over a median follow-up of 3 years, LVEF increased from 32.4 ± 8.9% at baseline to 55.9 ± 10.7% (p<0.001) and NYHA class improved from 2.73 ± 0.58 to 1.03 ± 0.18 (p<0.001). LBBB correction threshold remained stable with an acute threshold of 2.13 ± 1.19 V/0.5 ms and 2.29 ± 0.92 V/0.5 ms at 3-year follow-up (p>0.05). The high super-responder rate in this study was likely due to its highly selective study population of patients with typical LBBB and non-ischaemic cardiomyopathy.
Vijayaraman et al. reported a single centre observational series evaluating the feasibility of LBBP in 100 patients about 24% of whom had LBBB and 11% of total cases had a CRT indication.24 In patients with LBBB, the QRSd could be significantly narrowed from 162 ± 21 ms at baseline to 137 ± 19 ms during LBBP (p<0.001). At 3-month follow-up (n=68), the pacing thresholds and sensing remained stable at 0.68 ± 0.21 V at 0.5 ms (p=0.51) and 12.3 ± 5.7 mV (p=0.21), respectively. Only 11% of cases in this study had a CRT indication which limited the evaluation of LBBP for CRT.
Huang et al. reported a 97% success rate with LBBP in a prospective multicentre study involving 63 patients with non-ischaemic cardiomyopathy and LBBB.26 QRSd narrowed from 169 ± 16 ms to 118 ± 12 ms (p<0.001). Pacing threshold and R wave amplitude remained stable at 1-year follow-up (0.5 ± 0.15 V/0.5 ms versus 0.58 ± 0.14 V/0.5 ms and 11.1 ± 4.9 mV versus 13.3 ± 5.3 mV, respectively). LVEF increased significantly (33 ± 8% versus 55 ± 10%; p<0.001), with a reduction in left ventricular end systolic volume (123 ± 61 ml versus 67 ± 39 ml; p<0.001) and an improvement in NYHA functional class from 2.8 ± 0.6 at baseline to 1.4 ± 0.6 at 1 year. Again, the selective study population – patients with typical LBBB and non-ischaemic cardiomyopathy – likely resulted in a high rate of super-responders.
Wu et al. compared LBBP with HBP and BVP in a non-randomised observational study including 137 patients with non-ischaemic cardiomyopathy (49 HBP, 32 LBBP and 54 BVP).29 Mean paced QRSd was 100.7 ± 15.3 ms, 110.8 ± 11.1 ms and 135.4 ± 20.2 ms during HBP, LBBP, and BVP, respectively. HBP and LBBP demonstrated a similar absolute increase (Δ) in LVEF (+23.9% versus +24%, p= 0.977) and rate of normalised final LVEF (74.4% versus 70.0%, p=0.881) at 1-year follow-up. This was significantly higher than in the BVP group (Δ LVEF +16.7% and 44.9% rate of normalised final LVEF, p< 0.005). HBP and LBBP also demonstrated greater improvements in NYHA class compared with BVP. LBBP was associated with higher R-wave amplitude (11.2 ± 5.1 mV versus 3.8 ± 1.9 mV, p< 0.001) and lower pacing threshold (0.49 ± 0.13 V/0.5 ms versus 1.35 ± 0.73 V/0.5 ms, p<0.001) compared with HBP. Although non-randomised, this study demonstrated that both HBP and LBBP may be superior to BVP when evaluating echocardiographic response.
The largest retrospective multicentre study assessing the feasibility of LBBP for CRT was published by Vijayaraman et al in 2021.25 LBBB pattern was noted in 39% of this cohort, RBBB in 17% and intraventricular conduction defect in 15%. CRT was successfully achieved by LBBP in 277 of the 325 patients (85%) in which it was attempted and resulted in a significant reduction in QRSd from 152 ± 32 ms to 137 ± 22ms (p<0.01). LVEF improved from 33 ± 10% to 44 ± 11% (p<0.01) and was noted in both ischaemic and non-ischaemic patients. The lead threshold and R wave amplitude (0.6 ± 0.3 V at 0.5 ms and 10.6 ± 6 mV at implantation) remained stable during follow-up of 6 ± 5 months. Clinical response was noted in 72% and echocardiographic response in 73% of patients while 31% of patients were super-responders.
Patients with Right Bundle Branch Block and HFrEF
It has been demonstrated that BVP-CRT benefits patients with LBBB more than patients with RBBB or IVCD patterns. In patients with typical isolated RBBB, it is the RV that contracts asynchronously with normal LV activation. Hence, BVP-CRT may not correct this delayed RV activation and may not improve outcomes in patients with RBBB. On the other hand, in patients with correction of RBBB with HBP, delayed RV activation can be overcome while maintaining near normal LV activation.
Sharma et al. reported their multicentre findings with HBP in RBBB. HBP was attempted in patients with HFrEF and RBBB, with an overall success rate of 95% (37 of 39 patients) including complete correction of RBBB in 78% of cases.22 Over a mean follow-up of 15 months, a significant narrowing of QRS from 158 ± 24 ms to 127 ± 17 ms (p=0.0001), with an improvement in LVEF (31 ± 10% to 39 ± 13%) (p=0.004) and NYHA functional class from 2.8 ± 0.6 to 2 ± 0.7 (p=0.0001) was noted with HBP. His capture and BBB correction thresholds were 1.1 ± 0.6V and 1.4 ± 0.7 V at 1 ms, respectively. An increase in capture threshold occurred in three patients. This was the first multicentre observational analysis signaling that HBP might be an ideal strategy for CRT in patients with RBBB and HFrEF.
Patients with Pacing-Induced Ventricular Dyssynchrony and HFrEF
Conventional RV apical pacing leads to ventricular dyssynchrony which can predispose to PICM in a subset of patients. Recent data suggest that this risk can be as high as one in five patients among patients with an RV pacing burden ≥20%.30 CSP can help resynchronise ventricular activation thereby resulting in resolution of cardiomyopathy. Most of the studies evaluating this indication have evaluated HBP as an option for CRT. The multicentre findings on HBP for CRT by Sharma et al. included 31 patients with PICM with successful HBP in 81% patients (25 of 31).21 There was a significant decrease in QRSd from 177 ± 19 ms to 125 ± 15 ms (p=0.0001) and the LVEF increased from 32 ± 11% to 45 ± 13% (p=0.0001) in this subset.
Shan et al. demonstrated a success rate of 89% among the 18 patients undergoing attempted HBP upgrade, 11 of which were patients with PICM.31 In this study, the QRSd decreased from 157 ± 22 ms to 107 ± 17 ms, p<0.01. During a 1-year follow-up, left ventricular end-diastolic dimensions decreased from baseline 62 ± 7 mm to 56 ± 8 mm (p<0.01) and LVEF increased from 36 ± 8% to 53 ± 10% (p<0.01).
Vijayaraman et al. published a multicentre observational study with HBP in patients with longstanding AVB and chronic RV pacing and/or PICM in need of CRT.32 HBP was successful in 79 of 85 patients (93%) with chronic RV pacing. QRSd increased from 123 ± 31 ms at baseline to 177 ± 17 ms (p<0.001) during RV pacing and decreased to 115 ± 20 ms with HBP (p<0.001). HBP threshold was 1.47 ± 0.9 V at 1 ms at implant and 1.9 ± 1.3 V at 1 ms over a mean 2-year follow-up. In 60 patients with PICM in whom LVEF decreased from 54 ± 7.7% at baseline to 34.3 ± 9.6% (p<0.001) with RV pacing, there was an improvement of 48.2 ± 9.8% (p<0.001) after HBP.
Patients Undergoing Atrioventricular Node Ablation and HFrEF
Intuitively, CSP may be the best strategy for maintenance of ventricular synchrony in patients with permanent AF, narrow QRSd at baseline and HFrEF undergoing an AVN ablation. In patients with intact distal AV conduction, BVP-CRT creates dyssynchrony by activating endocardial RV and epicardial LV after an AVN ablation. On the other hand, CSP preserves synchronised ventricular activation by pacing the intact native conduction system distal to the site of AVN ablation.
Vijayaraman et al. published their initial findings of HBP among 42 patients undergoing AVN ablation and demonstrated a 95% success rate.33 HBP threshold at implant was 1 ± 0.8 V at 1 ms and increased to 1.6 ± 1.2 V at 1 ms during a mean follow-up of 19 ± 14 months. Patients with LVEF ≤40% at baseline demonstrated a significant improvement in LVEF (33 ± 7% to 45 ± 9%, p<0.001) while those with an LVEF >40% at baseline, demonstrated a preserved LVEF (56 ± 5% to 57 ± 7%, p=0.5) during follow-up.
Huang et al. similarly evaluated HBP with AVN ablation in 52 patients, half of whom had HFrEF.34 HBP and AVN ablation was successful in 42 patients (80.8%). Over a median follow-up of 20 months, the LVEF increased significantly from baseline in patients with HFrEF (n=20). NYHA functional status improved 2.9 ± 0.6 to 1.4 ± 0.4 after HBP in patients with a low LVEF. The number of patients who required diuretics for HF decreased significantly (p<0.001).
Wang et al. evaluated 86 consecutive patients with persistent AF and HF who had indications for ICD implantation and split them into patients receiving HBP/LBBP with ICD and AVN ablation (n=52) and the remaining patients underwent ICD implantation only (n=31).35 During follow-up, patients with HBP/LBBP and AVN ablation had a lower incidence of inappropriate shocks (15.6% versus 0%, p<0.01) and adverse events (p=0.011) and a higher improvement in LVEF and reduction in LV end-systolic volume (15% versus 3%, p<0.001; and 40 ml versus 2 ml, p<0.01, respectively).
The above studies have highlighted that HBP and LBBP are reasonable options for CRT for patients undergoing AVN ablation. However, the observational nature and lack of randomisation to conventional BVP-CRT are limitations of these studies.
Non-responders to Biventricular Cardiac Resynchronisation Therapy
Limited data exists on CSP in patients who have not responded to traditional BVP-CRT. In our multicentre study on HBP for CRT, we had eight patients who were deemed non-responders to BVP-CRT that underwent a successful upgrade to HBP.21 Six patients (75%) had an echocardiographic response with HBP with an average increase in LVEF from 30 ± 10% to 38 ± 13% (p=0.07).
Similarly, in the study by Shan et al. there were five patients who had successful upgrade to HBP with improvements in LVEF and NYHA functional class.31 Although these are interesting findings, the small sample size in these studies and the varied definition of non-response to BVP-CRT, this sub-group of patients needs further careful evaluation before CSP can be considered a standard approach.
Patients with Intraventricular Conduction Delay and HFrEF
Most IVCD patterns with QRSD <150 ms typically represent intra-myocardial cell-to-cell conduction delay and may not benefit from CSP given the lack of focal disease within the AV conduction system. However, in patients with advanced cardiomyopathy and dilated ventricles, AV conduction disease such as LBBB and intramyocardial delay may coexist. In these circumstances, resynchronisation may be more complete with pacing at the level of both the specialised conduction system (distal to site of AV delay) in conjunction with sequential LV pacing in areas of delayed myocardial activation, referred to as His-optimised CRT (HOT-CRT).
HOT-CRT was evaluated in a small series of patients by Vijayaraman et al.23 In this study, 27 patients with LBBB/IVCD in whom only partial or no QRS narrowing was achieved by HBP alone, underwent an LV epicardial lead implantation in addition to HBP. HOT-CRT resulted in improved electrical resynchronisation when compared with conventional BVP or HBP alone and was felt to be the best clinical option for these patients. The QRSd reduced from 183 ± 27 ms at baseline to 120 ± 16 ms (34%) with HOT-CRT compared to 162 ± 18 ms (11%) by conventional BVP (p<0.05). There was an observed significant echocardiographic and clinical improvement in 95% of patients with advanced HF treated with HOT-CRT.
Left Bundle Branch Pacing Versus Left Ventricular Septal Pacing
LBBP and LV septal pacing may be able to overcome more distal conduction disease, such as intrahisian disease, proximal BB disease, and might provide a better opportunity for synchronised LV activation than HBP in such cases. However, LV septal pacing alone versus LBBP (which involves capture and engagement of the left fascicular system) are mechanistically different in LV activation. How this affects resynchronisation, particularly in patients with LBBB and the need for CRT has been evaluated acutely in two studies.
The effects of temporary LV septal pacing without left fascicular system capture were studied in a canine model with LBBB and a human study with LBBB by the Maastricht group.36,37 Electrocardiography measuring QRSd, vectorcardiography measuring QRS area, and multielectrode body surface mapping, measuring standard deviation of activation times, were used to assess electrical resynchronisation. LV septal pacing resulted in a larger reduction in QRSd/area and LV activation time when compared to BVP or LV septal and RV pacing.37 Basal, mid- and apical LV septal pacing positions resulted in similar results, indicating that within the LV septum, the position of the pacing electrode is not critical. Changes in QRS area, LV activation time and LVdP/dtmax were comparable between LV septal and HBP suggesting that electrical resynchronisation and haemodynamic improvement with LV septal pacing may be as good as that with BVP or HBP in the short term.37
Anecdotally, LV septal capture might be reasonable for patients with normal or mildly depressed LVEF, but whether it truly adds value as a strategy for CRT in patients with HFrEF, particularly in those with severely depressed LVEF and a dilated LV cavity remains unknown. The long-term clinical benefit of LV septal pacing compared to HBP or LBBP, particularly in the CRT-indicated patient still needs further systematic evaluation.
During LBBP and LV septal pacing, RBBB delay pattern is often seen in varying degrees due to late activation of the RV. The implications of RV dyssynchrony likely induced by this RBBB pattern in patients undergoing CRT is unclear. However, this RBBB delay/dyssynchrony can be minimised by fusing with native RBB conduction using AV optimisation, bipolar pacing with anodal capture of RV septum, or fusion with RV or RBB pacing using another lead.
Proposed Algorithm for Conduction System Pacing
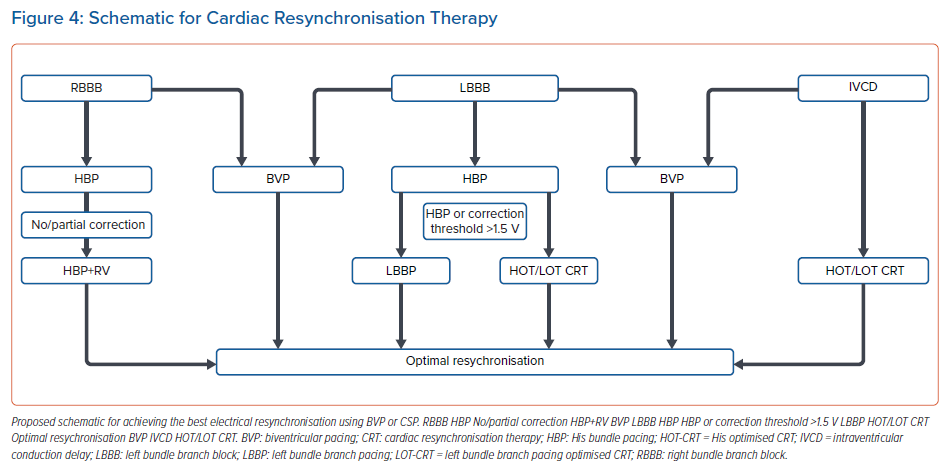
Figure 4 summarises a proposed algorithm for CRT using CSP. As noted above, BVP may not be the ideal resynchronisation strategy for patients with HFrEF and RBBB, and HBP likely provides the best option for biventricular activation. In cases with incomplete or no correction of RBBB pattern with HBP, RV pacing can be combined with HBP to pre-excite the RV and provide resynchronisation. BVP still remains the gold standard for CRT for patients with LBBB and HFrEF. CSP with HBP may be considered as a primary or rescue strategy in such cases. If HBP results in correction of LBBB with a correction threshold ≥ 1.5 V at 1 ms, LBBP should be considered. If HBP or LBBP result in partial correction, HOT/LOT-CRT may be performed by combining CSP with LV pacing. Similarly, for patients with an IVCD pattern, combining CSP with LV pacing may be an option. AV delay optimisation with any of these above strategies, particularly with LBBP, can result in further narrowing of the QRS width and help with RV activation.
Challenges with Conduction System Pacing
Despite a significant benefit and evolving indications for traditional BVP-CRT, limitations during implant such as the lack of suitable coronary sinus venous branches and unavoidable phrenic nerve stimulation at ideal anatomic LV lead positions can result in failure to achieve optimal CRT. CS lead dislodgement or increasing thresholds (if the lead is placed close to areas of scarring) can be issues in follow-up.
Similarly, both HBP and LBBP may not be successful in achieving CRT in all cases given the level of conduction system disease, presence of IVCD (myocardial conduction delay) and many cases with significant LV remodelling (with mixed conduction disease and myocardial delay).
In recent studies reported above, CSP has been reported to achieve successful CRT in 76–97% of cases. HBP may be associated with an increase in capture thresholds or loss of BBB recruitment thresholds in follow-up.
For instance, in the multicentre study on HBP for CRT by Sharma et al., an increase in HB capture threshold (defined as a >2 V increase in capture threshold from implant or capture threshold >5 V at 1 ms) was noted in seven (7.4%) cases, three of which resulted in loss of BBB recruitment.21 All of these occurred late during follow-up (>6 months postimplant) due to progressive increase in His capture threshold. Repeat procedures were needed in three patients (HBP extraction with manual traction and replacement with LV lead). Whether these increases are related to progression in conduction disease, microdislodgement of the lead (with cardiac motion) or lead-related properties remains unanswered. With LBBP, although thresholds have been reported to be stable during follow-up, long-term issues such as loss of LBB capture, lead dislodgement and the rarely reported complication of late LV septal perforation (into the LV cavity) have been reported.38
Future Directions
HBP and LBBP may provide a true physiological pacing strategy in patients with an intact distal His-Purkinje system. The above data suggest there is a potential value of this form of pacing for cardiac resynchronisation. CSP offers a promising alternative to BVP in non-responders or as a rescue strategy in those who fail BVP. While preliminary data from small, randomised, crossover studies suggest an equivalent response, it is important to emphasise that there have been no published large-scale randomised trials comparing the effectiveness of HBP or LBBP for CRT in comparison to BVP-CRT.
There are other unanswered questions to be considered:
- What is the degree of correction necessary to achieve electrical and mechanical resynchronisation with HBP or LBBP?
- Are there patient or ECG characteristics that can help predict patients who will achieve successful HBP and will respond to this therapy?
- Is one of these sites for CSP better than the other when looking at clinical outcomes?
- Is LBBP with left fascicular capture better/necessary for resynchronisation or is LV septal capture alone enough to improve outcomes in patients with HFrEF and LBBB?
Larger, multicentre, randomised studies are needed to evaluate the clinical efficacy of these strategies and help answer some of these questions.
Conclusion
CSP is a promising technique for ventricular pacing and helps maintain synchronous ventricular contraction. It can correct ventricular dyssynchrony in some patients and more data are emerging on the potential benefit of CSP for CRT. Larger randomised controlled trials and registry data can help realise the true benefit of CSP in comparison to BVP-CRT.
Clinical Perspective
- Conduction system pacing (CSP) which includes His bundle pacing and the left bundle branch pacing is a promising tool for patients who need ventricular pacing.
- CSP can also overcome bundle branch block patterns and thereby alleviate ventricular dyssynchrony.
- There are a number of studies demonstrating the clinical benefit of CSP in patients with heart failure, depressed LV function and known ventricular dyssynchrony.
- CSP might provide an additional modality for cardiac resynchronisation therapy in addition to conventional biventricular pacing.