Sudden cardiac death is a leading cause of global mortality, with 6 million deaths per year attributed to ventricular tachyarrhythmia.1 In at-risk groups, ICD improves survival, and is superior to medical therapy in both the primary and secondary prevention of sudden death.2–7 ICD therapy does not prevent arrhythmia, of course. Recurrence is common, even in the presence of anti-arrhythmic medications, which can themselves be associated with significant adverse effects.8–10 ICD shock therapies are also associated with increased mortality and reduced quality of life.11–14
Percutaneous catheter ablation is an effective and safe solution that can eliminate ventricular tachycardia (VT), reducing the risk of recurrent arrhythmia and future ICD therapies. In expert hands this can be achieved with low procedure-related complications and extremely low early mortality.15–20
Catheter Ablation: Indications
In patients with structural heart disease, expert consensus guidelines currently recommend catheter ablation for VT after failure of anti-arrhythmic drug (AAD) therapy, or for patients in whom AAD therapy is either not tolerated or not desired (class I indication).21
The most robust evidence for catheter ablation is in patients with both VT and ischaemic heart disease (IHD). VANISH was a randomised controlled trial in which patients with IHD and an ICD, who experienced VT despite AAD therapy, were randomised to either catheter ablation or an escalation in AAD therapy. After a mean follow-up of >2 years, a significant reduction in the combined primary outcome of death, ICD shock or VT storm was observed in the ablation cohort, with a subgroup analysis showing that patients who were already taking amiodarone obtained the greatest benefit.17 In the IHD population, catheter ablation is therefore recommended over escalation in AAD therapy for patients who experience VT despite amiodarone.
In patients with IHD, catheter ablation may also be considered after a first episode of monomorphic VT to reduce the future risk of shock therapies (class IIb indication). This recommendation is supported by the findings of SMASH-VT, a randomised controlled trial in which patients with a history of MI and a secondary prevention ICD indication were randomised to either ICD alone or ICD plus prophylactic catheter ablation. In this trial, appropriate ICD shocks were significantly reduced in the catheter ablation cohort with no increase in mortality.18
These findings were further supported by VTACH, a randomised controlled trial of ICD versus ICD and ablation, which recruited patients with prior stable VT, MI and reduced ejection fraction (<50%). Time to recurrence of VT or ventricular fibrillation (VF) was significantly longer in the ablation group (18.6 versus 5.9 months), while freedom from ventricular arrhythmia at 2 years was also significantly greater postablation (47% versus 29%).19
Ablation prior to ICD implantation is also a potentially promising strategy in patients with IHD, but this has not yet been shown to offer benefit over a deferred ablation strategy.20 However, we eagerly await the publication of on-going trials in this area, such as PARTITA (NCT01547208).16
In patients with non-ischaemic cardiomyopathy, catheter ablation is associated with lower rates of acute procedural success and higher rates of VT recurrence, when compared with ischaemic cardiomyopathy cohorts.22–24 In non-ischaemic cardiomyopathy, recurrence of VT postablation is dependent upon the underlying substrate, but may be reduced by early referral time after VT.25–27 Unfortunately, there remains an absence of randomised controlled data pertaining to VT management in non-ischaemic cardiomyopathy patients, although large case series of catheter ablation after failed medical therapy have shown that catheter ablation may reduce VT burden and result in greater discontinuation of AAD therapy.28 Catheter ablation of VT storm has also been shown to be effective, with recurrent storm rates as low as 5% after a median follow-up of 45 months.29
In idiopathic VT, catheter ablation is considered preferable to AAD therapy in symptomatic patients (class I indication) due to its high success and low recurrence rates.21 Catheter ablation may also be performed in patients who experience VT in the context of congenital heart disease, inherited arrhythmia syndromes or hypertrophic cardiomyopathy, and there is individualised guidance pertaining to each of these conditions within the guidelines.21
Approach to Catheter Ablation
There are two principal components to an effective catheter ablation procedure. The first is substrate identification, the process by which the critical substrate that is responsible for the initiation and/or maintenance of an arrhythmia is identified. Substrate identification requires an assimilation of all the available data, which might include pre-procedural investigations, the successful induction and mapping of a given VT circuit, and information obtained from invasive mapping during sinus rhythm. The second component is ablation, which requires sufficient energy to be delivered to the targeted tissue, ensuring permanent destruction and preventing further arrhythmia.
Substrate Identification
Pre-procedure
Substrate identification begins with a comprehensive pre-procedural assessment that is critical to the creation of an appropriate ablation strategy. This should include a considered review of any documented tachycardia. The 12-lead ECG is the most useful diagnostic tool in localising the origin of a VT and always requires scrutiny.30–32 Intracardiac electrograms (EGMs), downloaded from implantable cardiac devices, are also worthy of attention. These can provide useful information on VT morphology, cycle length, the relative timings at each ventricular lead and, where anti-tachycardia pacing has been delivered, the results of entrainment from the right ventricle.33–36 Prior ambulatory monitoring should also be reviewed, especially in the treatment of ventricular ectopy, where variations in ectopic burden can accurately predict the site of origin in outflow tract arrhythmia.37
Where possible, patients should have cardiac imaging prior to ablation, to ensure that the operator has a comprehensive understanding of the arrhythmia substrate prior to invasive mapping. Echocardiography is readily available, non-ionising and inexpensive, but multimodality cross-sectional imaging is also increasingly available and used in most centres. MRI with late gadolinium enhancement and CT can be used to localise and quantify scar substrate. Their usage pre-procedure has been shown to reduce both total procedure time and volume of ablation, as well as improve acute and long-term outcomes.38 Where there is no contraindication, cardiac MRI is routinely performed in our centre for all patients who present with ventricular tachycardia. Cross-sectional imaging may also identify potential technical challenges relating to either a proposed epicardial puncture or to obtaining retrograde ventricular access; for example, in the presence of significant peripheral vascular disease.
We believe that the need for epicardial access should be decided prior to ablation to ensure appropriate patient consent and to plan a suitable peri-procedural anticoagulation strategy. In general, epicardial ablation should be considered where epicardial or mid-myocardial substrate is anticipated from either cross-sectional imaging or prior invasive mapping, or where the presenting ECG suggests an epicardial VT exit. Although, patient-specific factors, including body habitus and prior cardiac surgery, must also be considered.
In patients with coronary artery disease, an endocardial-only approach for first-line ablative therapy has been favoured in our centre, due to the absence of randomised controlled data to support a more invasive initial strategy. Retrospective observational data from a small study (n=15 in the combined endo-epicardial ablation group) did show that an initial combined procedure resulted in fewer readmissions for VT and fewer repeat ablations, but we have not routinely adopted this approach.39
Conversely, in patients with non-ischaemic cardiomyopathy, where the prevalence of epicardial substrate is much higher, we have adopted an endo-epicardial ablation approach as first line. In patients with arrhythmogenic cardiomyopathy, this strategy is supported by a recently published meta-analysis in which significantly higher freedom from recurrent arrhythmia or ICD therapies (84.6% versus 52.2%) and greater elimination of AADs (69.2% versus 21.7%) was observed in the combined ablation group through >3 years of follow-up.40
Invasive Voltage Mapping
In the catheter laboratory, substrate identification is achieved through careful three-dimensional electroanatomical mapping using a dedicated mapping system. This can be achieved with a point-by-point approach, using an ablation catheter with or without the addition of contact force sensing, or through the use of a multi-electrode catheter.
Multi-electrode catheter mapping produces higher-density maps, and has been shown to provide better discrimination of late potentials in randomised trials.41 A meta-analysis has also shown multi-electrode mapping to be associated with a reduction in mapping time, but does not reduce procedure or ablation time, nor confer improvements in acute procedural success or VT recurrence.42 Contact force sensing is also not associated with an improvement in hard endpoints, although retrospective trial data suggest a reduction in fluoroscopy time.43
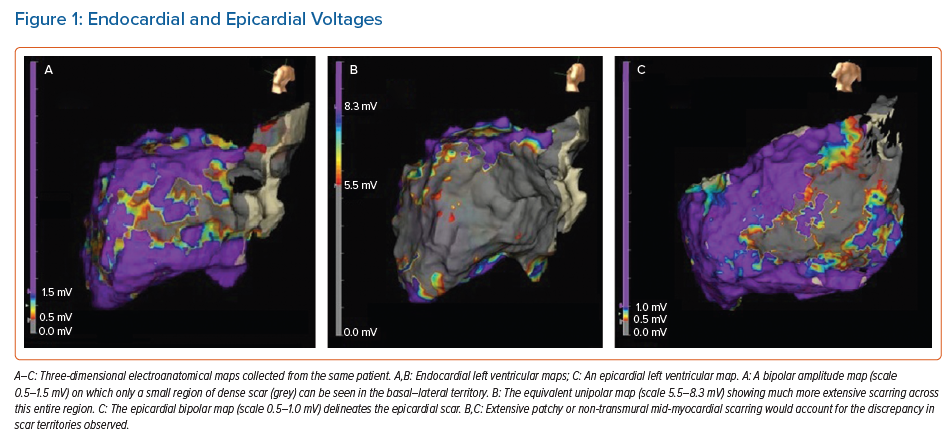
The amplitude of local EGMs is used to differentiate normal tissue from scar tissue, with automated bipolar and unipolar voltage maps created simultaneously, and displayed on three-dimensional electroanatomical maps (EAM). Normal endocardial bipolar voltage, measured between two adjacent electrodes at the endocardial surface, is generally accepted as being >1.5 mV, with dense scar identified by bipolar voltages of <0.5 mV.44,45
However, voltage limit adjustment may be required to accurately identify VT channels with relatively higher voltages (within regions of low voltage scar).46 The direction of myocardial activation has also been shown to alter the voltage characteristics of myocardial scar.47 In this study by Tung et al., separate voltage maps were created using different wavefronts of activation (right ventricular pacing, left ventricular pacing, intrinsic conduction) and approximately 18% of critical sites for re-entrant VT were found in regions that displayed low voltages during one wavefront of activation, and normal voltage (>1.5 mV) during another wavefront of activation.47
A possible future solution might be the use of omnipolar EGMs in creating ventricular EAMs. These represent a new approach to voltage mapping, taking advantage of the directional properties of the electric field of a travelling wavefront on the surface of the myocardium. Preliminary studies have shown that omnipolar EGMs are orientation-independent, effectively virtual bipolar EGMs that are aligned along the direction of a wavefront.48 Ventricular mapping with omnipolar EGMs has subsequently been achieved in animal models, but the clinical application of this technology to ventricular tachycardia ablation remains unclear.49
Unipolar voltages are recorded between a single mapping electrode and a neutral electrode sited away from the myocardium. Unipolar maps, therefore, have a greater ‘field of view’, which can facilitate the identification of mid-myocardial and epicardial scar. Defining ‘normal’ references for unipolar voltage is challenging, and flexibility is required when interpreting unipolar maps (Figure 1). In our centre, we loosely use the cut-off values of >8.3 mV in the left ventricle and >5.5 mV on the right ventricular free wall to define normal unipolar amplitudes.50,51 However, in the presence of endocardial scar, a lower cut-off value is often required, while a higher upper limit of normal may be required in the presence of significant left ventricular hypertrophy.
In the future, it may be possible to adopt more disease-specific unipolar reference ranges. Recent trials in both Chagas’ disease and remodelled post-infarct myocardium have suggested a shift away from standardised ranges to ensure accurate identification of substrate.52,53
Mapping During Ventricular Tachycardia
In the absence of spontaneous arrhythmia, programmed electrical stimulation is routinely used to induce VT. Where this is sustained – and occurs without haemodynamic collapse – activation and entrainment mapping can be performed. Unfortunately, approximately 60–70% of scar-related VT is associated with haemodynamic collapse.54,55 This has led to an increased interest in entirely substrate-based ablation, which has the potential benefit of identifying future substrate, at the expense of potentially unnecessary ablation.
The latest randomised controlled data support a substrate-based approach in patients with ischaemic heart disease and stable VT. When compared with a strategy in which only mappable VTs were targeted, the substrate-based group experienced less recurrent VT, required less AAD therapy and had fewer hospitalisations. There was also no difference in peri-procedural complications or 12-month mortality.56 A subsequent meta-analysis of six studies further supported the idea of complete substrate modification, with a significantly lower risk of the composite endpoint of arrhythmia recurrence and all-cause mortality, although more ablation was associated with a higher rate of complications.57
Mechanical circulatory support devices may be considered to maintain haemodynamic stability and preserve end organ perfusion while mapping sustained VT. Options include intra-aortic balloon pumps, atrial to femoral bypass systems, aorta-flow assist systems and extracorporeal membrane oxygenation.58 Observational data suggest that haemodynamic support can facilitate extended mapping, but at a cost of increased complications.59–61 To date, there are no randomised data to evaluate the efficacy of these strategies. Appropriate patient selection also remains challenging, although scoring systems have been developed to predict the risk of haemodynamic decompensation during VT ablation.62
Interestingly, general anaesthesia does not appear to reduce VT inducibility in the majority of patients, but it is associated with a greater need for haemodynamic support.63 Induction of VT under conscious sedation, rather than general anaesthesia, is therefore also worthy of consideration in cases where haemodynamic collapse is likely to prevent mapping in tachycardia.
In our centre, mapping of unstable tachycardias is frequently supported by pressor pre-loading and the strategic placement of multipolar catheters at the sites of interest prior to VT induction. This allows for a short period of mapping in tachycardia (~30 s), which can be sufficient to adequately identify critical substrate.
Activation Mapping
In activation mapping, the overall pattern of ventricular myocardial activation is determined from the timing of local EGMs and referenced to a consistent point in time (e.g. the onset of the QRS complex). This process can be largely automated using EAM software.
In a focal ventricular arrythmia, the site of earliest activation is targeted for ablation, with local EGMs >24 ms prior to the earliest activation of the surface QRS a predictor of long-term success.64 Characteristically, the local EGM may also display a reverse polarity pattern on the distal and proximal bipoles of the ablation catheter, often with low amplitude initial components.65 However, this finding is not specific, and pseudo-focal patterns may also be present in deep substrate.
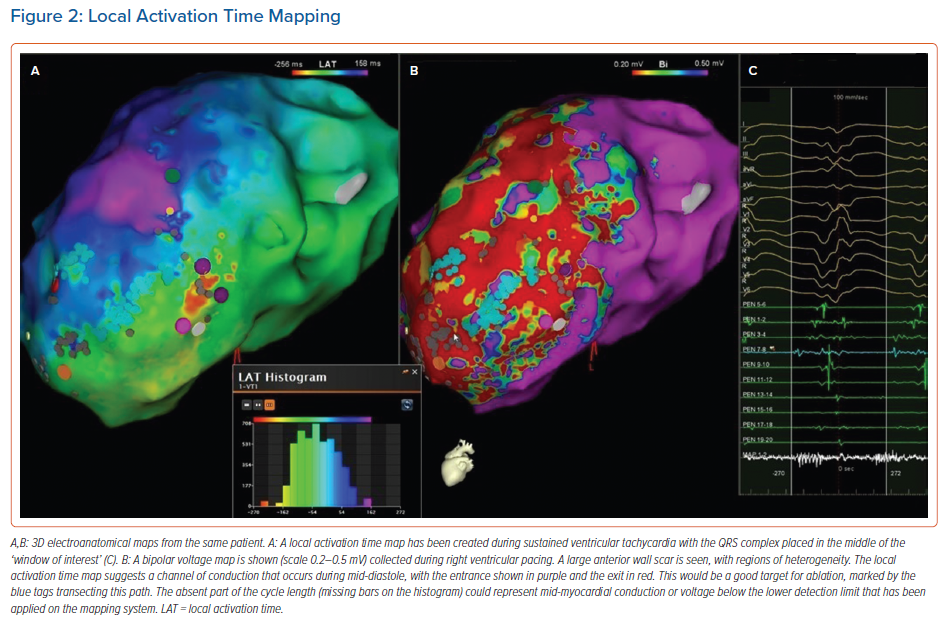
In re-entrant VT, activation maps require a different interpretation, as there is no focal activation point, but a continuous circuit of electrical activity (Figure 2). Local EGM signals that are immediately pre-QRS represent the point at which the wavefront exits the protected isthmus into the myocardium, while electrical activity through the critical isthmus is identified by the presence of mid-diastolic potentials. However, passive slow conduction is common within scar tissue, and mid-diastolic activation alone is not sufficient to determine active involvement in the re-entry circuit. The critical components of the re-entry circuit can therefore only be accurately distinguished by entrainment.
Entrainment Mapping
Entrainment is a manoeuvre in which pacing is delivered at a constant cycle length shorter than that of the tachycardia, but not excessively short, as this might alter the conduction properties of the critical substrate and lead to inaccurate information. The aim is to continuously reset the arrhythmia, but without termination, so that it continues, unchanged, on cessation of pacing. Observations made during and after entrainment are crucial to understanding the arrhythmia mechanism and in locating the critical substrate.
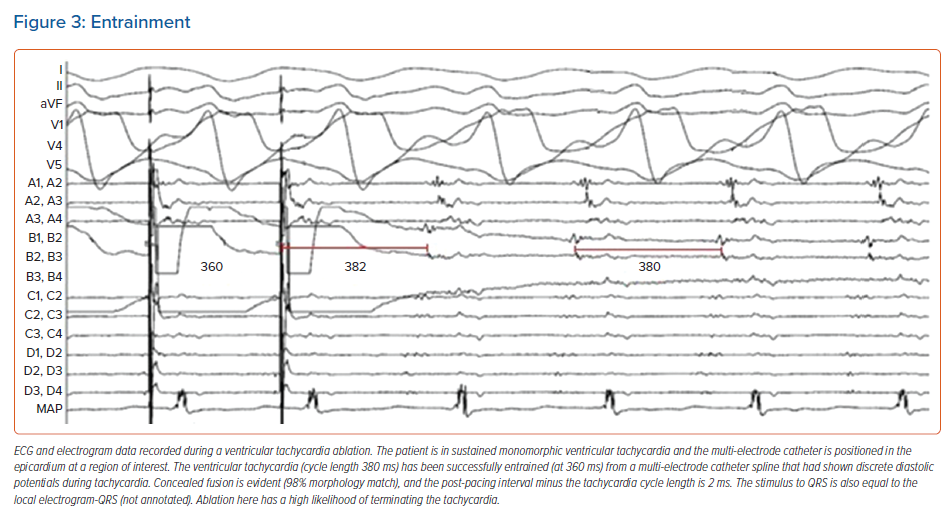
In scar VT, entrainment from the protected critical components of the circuit (critical isthmus and exit) results in a QRS morphology that is identical to that of the VT (entrainment with concealed fusion). By comparison, entrainment from elsewhere in the ventricle results in manifest fusion of the QRS complex. On cessation of entrainment, the postpacing interval minus the tachycardia cycle length can also be used to determine the distance between the pacing catheter tip and the protected circuit, with critical sites expected to have a postpacing interval minus the tachycardia cycle length of <30 ms (Figure 3).66
Additionally, the stimulus to QRS time that is observed during entrainment with concealed fusion can be used to further subclassify the circuit components. At the critical isthmus, a stimulus to QRS interval of 30–50% of the tachycardia cycle length is expected. Furthermore, the stimulus to QRS interval should be close in duration to the local EGM-QRS interval observed during activation mapping. The critical isthmus is the commonest target for ablation, and the point at which ablation is most likely to terminate the rhythm and prevent its recurrence.67
Substrate Mapping Without Sustained Ventricular Tachycardia
Pace mapping
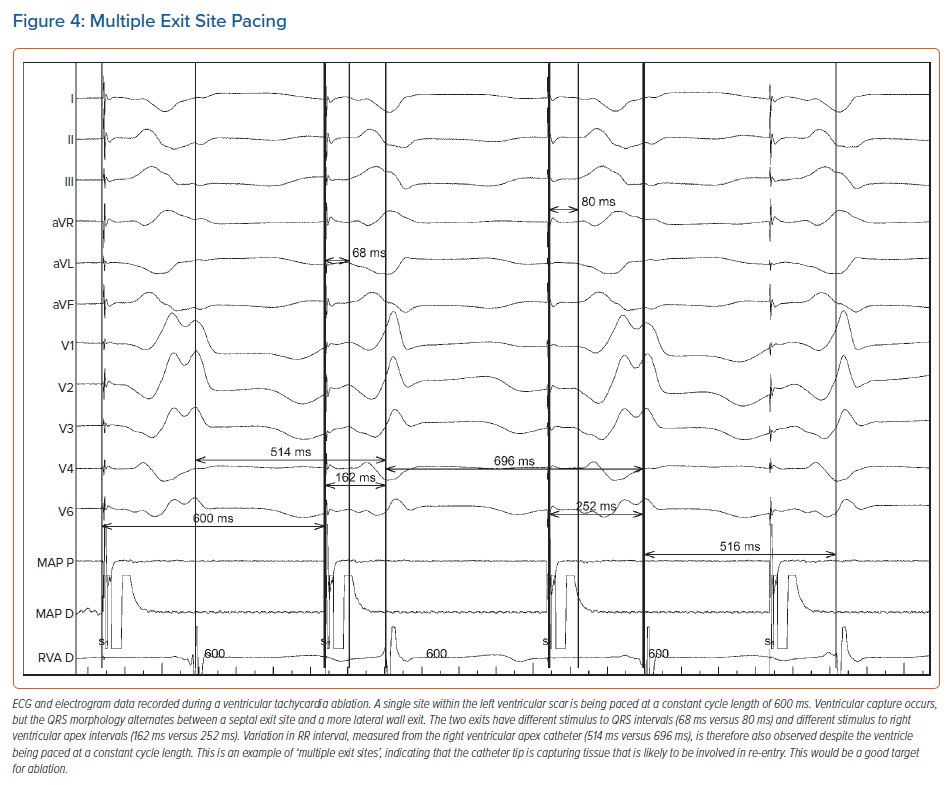
Pace mapping can be used to assist with identification of the site of origin of a focal arrhythmia or the exit site/distal protected isthmus of a re-entrant circuit, but prior documentation of the clinical VT (or premature ventricular complexes) morphology is required. Pace mapping can also assist with the identification of substrate critical to the maintenance of re-entry (Figure 4).
The fundamental principle behind pace mapping is the positive correlation that exists, when healthy tissue is paced, between QRS morphological change and the distance the pacing catheter tip is moved. This allows a quantitative assessment of the pace mapping output, a process that can be automated by EAM software.68
However, adjacent tissue capture due to high pacing output in bipolar configuration, and possibly saline irrigation, may lead to alterations in QRS morphology, which might limit the accuracy of pace mapping. Consequently, some centres resort to unipolar pacing to minimise the degree of local tissue capture. Theoretically, wavefront activation during VT might also differ from a paced QRS from a perfect exit site, due to the absence of refractory myocardium that is only present during tachycardia, although this is not usually encountered clinically.
Recently, pace mapping has also been used to unmask VT isthmuses in patients with postinfarct re-entrant VT.69 In that study, the best pace map percentages were found, as expected, at the isthmus exit, while the poorest percentage pace maps were found adjacent to the scar border in the entrance zones and in the entrance part of the isthmus. On high-density 3D pace maps, an abrupt change in pace map match percentage was therefore found to be associated with the central isthmus, matching the location of the wavefront identified on activation mapping.
Local Abnormal Ventricular Activities
Local abnormal ventricular activities are sharp, high-frequency ventricular potentials, which are usually low in amplitude and arise from pathological tissue.70 Importantly they are distinct from, and can therefore be uncoupled from, the larger far field ventricular EGM within which they reside or follow. They can be identified using a standard ablation catheter or more readily using a multi-electrode mapping catheter either during sinus rhythm or ventricular pacing. Elimination of all local abnormal ventricular activities provides a clear and reproducible end point for ablation and has been reported as both feasible and safe. Furthermore, where local abnormal ventricular activity can be identified, elimination is independently associated with superior survival from recurrent VT, although this has not been prospectively assessed in a randomised controlled trial.70
Late Potentials
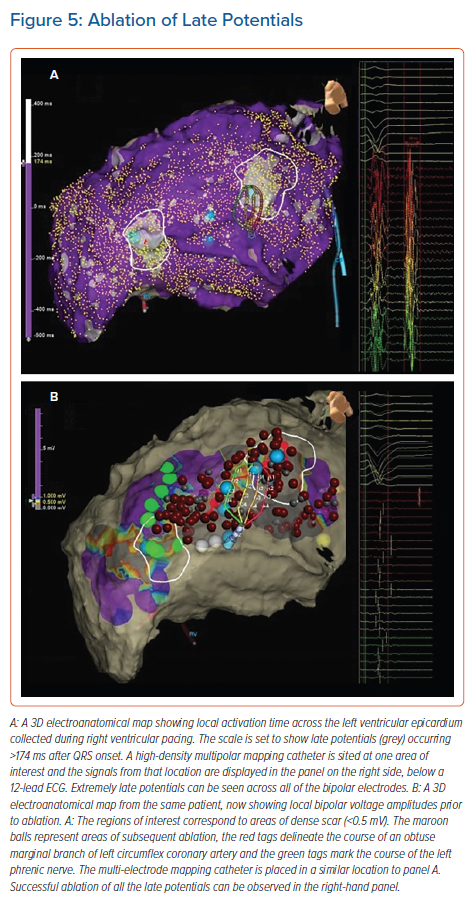
Late potentials are local EGMs that occur after the terminal portion of the surface QRS, either entirely isolated from other local activity or due to continuous fractionation. They are present in the majority of patients who have had a MI, and can be easily identified when mapping during sinus rhythm (Figure 5).
The elimination of all late potentials is a potential endpoint for a substrate-based ablation strategy. In a large study of ischaemic patients in whom the combined endpoint of non-inducibility and the elimination of all late potentials was employed, VT recurrence was reduced to low levels and a significant reduction in cardiac death was observed. However, this was not a randomised controlled trial.71
Scar Dechannelling
Scar dechannelling is a technique by which a scar is identified using standard bipolar voltage parameters, but then further EGM analysis is undertaken to identify the various entry points into this delineated scar. These channels can then be eliminated by ablation at the entry points, with a view to abolishing a potential isthmus from this territory.
This mapping technique requires software that can identify abnormal or delayed components on a local EGM (usually fractionated and low voltage) that are only marginally delayed with respect to the healthy far field component of the EGM (usually higher voltage and lower frequency). These signals are felt to represent scar channel entry points.
Scar dechannelling has been shown to result in low rates of VT recurrence and mortality, as well as limiting the extent of ablation required in more than half of patients. Incomplete channel elimination was also associated with higher VT recurrence rates.72 Cardiac MRI-aided scar dechannelling, where MRI with late gadolinium enhancement tissue characterisation is integrated into the navigation system, has also been associated with a lower need for radiofrequency (RF) delivery, higher non-inducibility rates after substrate ablation and a higher VT recurrence-free survival.73
Functional Substate Mapping
Slow conduction and conduction block are pre-requisites for re-entry, and tissues that display these characteristics represent regions of interest in the hunt for critical VT substrate. Conduction velocity and conduction block are not fixed characteristics of a tissue, they depend upon the timing and direction of depolarising wavefronts. As such, functional tissue assessments can provide greater substrate characterisation than a purely voltage-based approach. In recent years, this has been the subject of significant research, and several advanced mapping techniques have emerged.
Isochronal Late Activation Maps
Isochronal late activation maps, produced in sinus rhythm or during constant ventricular pacing, have been proposed as a possible supplementary technique for identifying critical ablation targets. Isochronal late activation maps are readily created using EAM software. Local EGMs are timed at the latest bipolar component, signifying the completion of local activation, and displayed across equally distributed isochrones. Where conduction velocity slows, isochrones are observed to crowd together, and ‘deceleration zones’ can be identified. Sites critical to re-entry have been retrospectively shown to occur in these regions.74 VT ablation guided by the production of isochronal late activation maps has also been performed prospectively with successful termination sites found to colocalise to the deceleration zones in 95% of cases, with high rates of freedom from VT at 12 months.75
Decremental Evoked Potential Mapping
Decrement evoked potential (DEEP) mapping involves stressing possible substrate tissue to identify local conduction delay and unidirectional block, both of which are pre-requisites for re-entry. DEEP mapping was first described using a ventricular drive train followed by a single ventricular extrastimulus, delivered 20 ms above the refractory period of the ventricle.76 The response of local late potentials to this extrastimulus was mapped using EAM software. The potentials that were significantly delayed (>10 ms) in comparison with their relative timing during the drive chain were proposed as possible targets for ablation. However, an important limitation of DEEP mapping is the potential for proximal slowing of conduction to delay a downstream EGM, the critical site of delay thereby going undetected.
This DEEP mapping technique was assessed in a small, non-randomised study (n=20) in which all identified late potentials were interrogated by the introduction of an extrastimulus. Critical VT substrate was identified with high specificity, allowing for a more focused ablation strategy, and the majority of patients were rendered non-inducible for clinical VT.77
DEEP mapping has also been assessed using a sensed extrastimulus approach, removing the need for a drive train, facilitating a shorter mapping time.78 In this small (n=30), non-randomised study, ablation guided by a right ventricular sensed extrastimulus protocol rendered VT non-inducible in 97% of patients, whereas 90% remained free from both symptomatic VT and ICD therapies at a median follow-up of 12 months.78
Substrate Mapping for VF
The ablation of VF most commonly involves the targeting of triggering premature ventricular complexes. These typically arise from the Purkinje system or scar border zone, and are characterised by short QRS duration and a short coupling interval. As with all ventricular ectopic ablation, they can be localised by a combination of activation mapping and pace mapping. In patients with a history of VF, the ablation of triggering ectopics has been shown to reduce recurrent VF in patients with ischaemic cardiomyopathy, Brugada syndrome, long-QT syndrome and early repolarisation syndrome.79–82
In the absence of triggering premature ventricular complexes, the ablation of low amplitude EGMs with fractionation or late potentials has been shown to reduce recurrent VF in patients with and without structural heart disease.83 In patients with idiopathic VF, empirical ablation of the left posterior fascicle, targeting the Purkinje potentials seen along the left ventricular inferoseptum, has also been shown to be of benefit in a small case series.84 An empiric strategy of interrogating the Purkinje’s network, papillary muscles and outflow tract regions by pace map matching against stored ICD template EGMs has also been shown to reduce arrhythmia burden and AAD usage.85
In patients with early repolarisation syndrome and recurrent VF, non-invasive electrocardiographic imaging has also been used in the identification of targets for ablation. Electrocardiographic imaging showed potential in identifying regions of abnormal conduction (delayed activation, conduction block, fractionation) and abnormal repolarisation.86 In a small, non-randomised trial, this technique, in combination with tradition electroanatomical mapping, has also been shown to result in effective ablation in symptomatic patients with recurrent VF.87
Recently, there has also been an increased interest in the identification of rotors and wavelets that might maintain ventricular fibrillation. A 64-electrode basket catheter has recently been used to obtain a substrate map during VF.88 In that small non-randomised study (n=6), patients with drug refractory VF had areas of localised re-entry identified during VF. Ablation of these targets resulted in a significant reduction in ICD shocks and all-cause mortality compared with a non-ablation control group.
Ablation Techniques
RF catheter ablation requires sufficient energy to be delivered to the targeted tissue to ensure permanent elimination of local conduction. In the early days of VT ablation, conventional electrode catheters were found to be ineffective in the ventricle, with the most likely explanation being the relatively small and shallow lesions they created.89,90 Larger irrigated-tip catheters have since become the routine standard of care.
Irrigation of room temperature saline at the catheter tip has been shown to reduce the electrode tip temperature, decrease the likelihood of thrombus and char formation, and result in larger and deeper lesions.91 Catheters with an 8 mm tip have been shown to have greater efficiency in the treatment of ventricular arrhythmias than 4 mm tipped catheters, but even with modern irrigated catheters, effective energy delivery can still be challenging.92 For example, when the arrhythmic substrate is located in areas that are difficult to access percutaneously (left ventricular summit or intra mural substrate), or where energy delivery is impeded by epicardial fat, coronary vessels or scar calcification. At all times, the potential benefit of delivering higher energy to the tissue must be balanced against the increased risk of complications.
Alternative Irrigation Solutions
In recent years, the use of alternative irrigants has provided a possible solution to these challenges. Solutions with lower ionic concentrations and charge densities have been shown to result in larger RF lesion volumes, as the increased impedance around the catheter tip decreases the dissipation of energy into the blood pool, maximising the energy conducted to the tissue.93
Half normal saline has been prospectively assessed as an alternative irrigant in a multicentre trial of 94 patients with ventricular arrhythmia refractory to standard ablation techniques. Half normal saline was found to be safe and effective, with acute rhythm suppression achieved in 83% of cases.94 Dextrose irrigation has also been trialled in an ovine ventricular model with lesion sizes comparable to those achieved with half normal saline, both of which were larger in volume than normal saline irrigated lesions.95
As alternative irrigation solutions enhance energy transfer into the tissue, operators should be mindful of excessive tissue heating deep to the surface and the possibility of precipitating steam pops. In the aforementioned trials, steam pops were more frequently observed with both alternative irrigation solutions, more so with the use of dextrose, although without adverse consequence.
Bipolar Ablation
During RF ablation, the dispersion of energy is proportional to the distance from the catheter tip. Unipolar lesions are therefore limited to approximately 5–6 mm in depth, even with high power settings. This may be troublesome when the critical substrate is located deep in the tissue, rather than at the endocardial surface.
One possible solution is bipolar ablation, where current is applied between two ablation catheter tips that are positioned on either side of the targeted tissue. This approach has been prospectively tested in both non-ischaemic cardiomyopathy patients with an intraseptal substrate (n=21), and in patients with deep intramural substrate that could not be suppressed by unipolar RF (n=18). Non-inducibility was achieved in 89–95% of these cases with an excellent safety profile.96,97
We strongly consider this technique in our centre for patients requiring redo procedures and those with documented septal substrate on imaging. Direct loss of pacing capture from a diagnostic catheter strategically placed in an intramural septal vein, in close proximity to a scar, has also been used to facilitate – and acutely demonstrate the value of – bipolar ablation in targeting deep septal substrate.98
Retrograde Coronary Venous Ethanol
RF ablation of VT can fail because of inaccessibility of the critical substrate. In left ventricular summit VTs, for example, the critical substrate might only be identified on mapping of the coronary sinus and its tributaries. Retrograde coronary venous ethanol using an angioplasty balloon, to ensure retrograde flow of the ethanol, is a possible solution.99
In a prospective multicentre trial, retrograde coronary venous ethanol has been shown to be safe and effective, offering long-term control of drug and RF-refractory ventricular arrhythmia. Isolated or adjunctive retrograde coronary venous ethanol was successful in 98% of patients (n=56), 77% of whom remained free of recurrence at 12 months.100
Future Developments
There are several novel ideas in development that may significantly influence ventricular ablation in the coming years.
Needle Catheter Ablation
A novel RF catheter has been developed with an extendable and retractable 27-G needle that can be used to target deep intramural substrate. This technology is not presently available commercially, but a single study of 31 patients demonstrated control of otherwise refractory arrhythmias, with acceptable procedural risk.101 In a porcine model, this idea has been further developed to include an extendable infusion needle electrode capable of delivering warm saline directly to intramural tissue. This has been shown to cause near transmural lesions with no wall thinning or perforation, but has not yet been tested in humans.102
Pulsed Field Ablation
Pulsed field ablation (PFA) is a novel, non-thermal modality that selectively ablates myocardium with ultra-short electrical impulses that create microscopic pores in the cell membrane, known as electroporation. Unlike thermal ablation with RF, tissue-specific thresholds exist for electroporation, allowing for selective ablation of the cardiac myocytes. This was demonstrated in animal trials, where PFA created durable myocardial lesions with no discernible oesophageal damage.103–105
First-in-human trials of PFA have already been conducted in patients with AF. Ultra-rapid pulmonary vein and left atrial posterior wall isolation have been demonstrated using a single shot PFA catheter. Furthermore, combined RF and PFA ablation has also been performed using a novel lattice-tip catheter capable of delivering either technology.106–108 In each of these trials, excellent safety profiles and lesion durability were demonstrated.
A proof-of-concept study of endocardial ventricular PFA using porcine myocardium has also now been reported, with homogenous myocardium-specific lesions successfully created.109
PFA appears to yield much promise, although the technology remains in its infancy and patient numbers remain very low. Long-term data on both safety and efficacy is also awaited, although further trials are anticipated for both atrial and ventricular ablation.
Stereotactic Body Radiation Therapy
Radiotherapy is a long-established therapeutic technique in which high-energy X-rays, gamma rays or a photon beam are targeted to abnormal tissue, most commonly cancer cells. In recent years, attention has turned to the use of radiotherapy as a possible treatment for ventricular arrhythmia, after myocardial irradiation was shown in animal models to create transmural fibrosis, similar to the results of catheter ablation.110,111
Both invasive and non-invasive techniques can be used to characterise the VT and localise the arrhythmic substrate as accurately as possible prior to radiotherapy. In a prospective case series of five patients, high-density surface electrocardiographic data from a 252-electrode vest was used to characterise the VT, combined with a chest CT scan to delineate the substrate using an entirely non-invasive approach.112 Post-therapy, a 99.9% reduction in overall VT burden was observed.
Longer-term follow-up data are now available from two prospective study cohorts. Safety and efficacy are demonstrated at >12 months, with reductions in VT burden and anti-arrhythmic drug therapy, as well as improvements in quality of life.113,114
These results are promising, but the evidence is restricted to case reports and small case series (both prospective and retrospective), in which the majority of recipients have failed standard ablation or been deemed unsuitable for invasive therapy. A randomised clinical trial is needed. It also remains to be seen what the potential application of this technology could be in less frail patients, and whether it would supersede standard ablation techniques.
Conclusion
Catheter ablation is a safe and effective treatment strategy for VT, for which increasingly sophisticated techniques in both mapping and ablation have been recently developed. Contemporary VT ablation requires a comprehensive understanding of the principles of both patient preparation and invasive substrate mapping, to which additional and more novel techniques can then be appropriately applied.
Future advances, with the promise of reducing procedural risk and duration, and improving outcomes, are also eagerly anticipated.
Clinical Perspective
- Percutaneous catheter ablation is a safe and effective therapy that reduces recurrent ventricular tachycardia (VT) and shock therapies where anti-arrhythmic drugs have failed or not been tolerated.
- Prior to VT ablation, a thorough pre-procedural assessment is required, and should include a considered review of prior tachycardia and cross-sectional imaging.
- Haemodynamic stability during VT largely determines the methodology by which critical substrate is identified, with increasing evidence to support an entirely substrate-based approach where needed.
- Novel physiological mapping and advanced ablation techniques are increasingly used to supplement the traditional techniques of activation, entrainment and pace mapping.
- Ventricular ablation is a rapidly progressing field with several new technologies and ideas that may change the therapy landscape in the coming years.