Numerous large clinical trials have demonstrated the benefit of implantable cardioverter-defibrillators (ICDs) to prevent sudden cardiac death (SCD) in selected populations.1,2 These results and advances in defibrillation therapies have led to an impressive expansion of ICD implants and indications in recent decades. Although initially focusing on secondary prevention, current ICD indications have expanded to include prophylactic implantation in individuals at high risk of SCD (primary prevention), increasing the potentially eligible ICD candidates.2–4
The introduction of transvenous ICDs (TV-ICDs) was a paradigm shift in ICD therapy by avoiding the surgical approach and its associated risks and comorbidities.5,6 Nevertheless, TV-ICDs are still associated with considerable morbidity (1.5 % major complications)7 and acute and long- term procedural risks.8–10 Late infections including endocarditis, vessel occlusion, lead dislodgment, valvular dysfunction and intrinsic lead defects with consequent inappropriate/ineffective therapies are also observed with endocardial leads.11 Notably, the TV leads have been considered the weakest link in the TV-ICD with up to 20 % annual lead failure rates for 8-year-old systems.12–14 Moreover, lead extraction of failed/infected chronic TV leads is a complex procedure requiring special skills and equipment, and it is also associated with substantial comorbidity and mortality.15,16
An ICD system residing only in the subcutaneous space, without touching the heart or its vessels, may further simplify the implant procedure and minimise the shortcomings related to the TV leads.
Conceptualisation of the Subcutaneous Defibrillation and Early Clinical Studies
The concept of a subcutaneous ICD (S-ICD) is not new. As early as in 1970, Schuder et al.17 reported on an implantable system provided with subcutaneous transthoracic electrodes in a canine model. In the successive decades, this concept was overlooked with the expanding use of epicardial, and subsequently, TV-ICD systems. In the early 2000s, a few cases of subcutaneous defibrillation were reported in children with venous access issues, but without applying a dedicated functional S-ICD.18–20
At the beginning of this century, growing knowledge and evidence of the shortcomings/risks related to the TV leads stimulated the investigators to conceive an entirely S-ICD system without touching the heart or its vessels. The initial challenge was to find out the optimal shock configuration regarding the positions of the subcutaneous lead and pulse generator (PG). The first short-term defibrillation trial was conducted in 78 patients to assess defibrillation threshold, according to different configurations of a temporary S-ICD system.21 The results led to the selection of the S-ICD shock configuration currently available for clinical use, consisting of a left lateral PG positioned at the fifth intercostal space between the mid and anterior axillary lines, and an 8 cm coil electrode positioned parallel to the left parasternal margin. Throughout 2004 and 2005, a second trial was conducted in 49 patients, with the aim of comparing the efficacy of an S-ICD shock configuration with that of a conventional TV-ICD system. Both subcutaneous and TV systems effectively defibrillated induced ventricular fibrillation (VF) in all but one patient, with a mean threshold of 37±20 J and 11±9 J, respectively. From late 2008 to early 2009, a multicentre clinical trial of permanent S-ICD implantation was conducted in New Zealand and Europe, involving 55 patients with class I, IIa or IIb indications for ICD therapy. Exclusion criteria included a clinical indication for antibradycardia pacing, severe renal insufficiency and a history of slow (<170 bpm), or pace-terminable ventricular tachycardia (VT). The S-ICD successfully detected and defibrillated (at 65 J) the induced VF in 100 % and 98 % of patients, respectively. The mean time to shock delivery was 14.0±2.5 seconds, whereas the mean procedure duration was 67±33 minutes. After 10±1 months of follow-up, a total of 12 episodes of clinical ventricular arrhythmia were detected and terminated effectively by the S-ICD. Minor complications were observed in five patients (pocket infection in two patients, parasternal subcutaneous lead dislodgement in three patients). Oversensing and inappropriate sensing were rare (double counting, muscle noise) and were managed by device reprogramming.
The S-ICD System
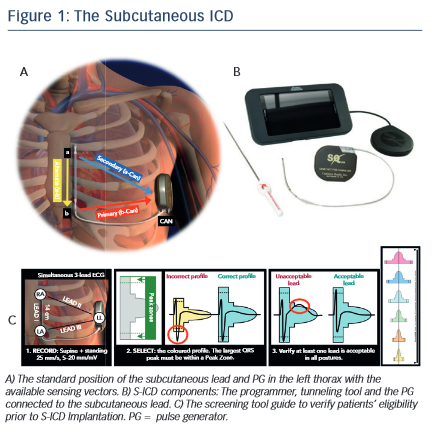
The system consists of a subcutaneous PG and a subcutaneous lead placed along the left side of the sternum. The first PG generation (model SQ-RX 1010, Cameron Health, Inc.) has a volume of ~70 cc, a weight of 145 g and a projected longevity of 5 years. The subcutaneous lead is provided with two sensing electrodes separated by an 8 cm shock coil. Using these sensing electrodes and the generator itself as the third one, three sensing vectors are available to detect the subcutaneous signals. The best vector is automatically selected by the system in order to avoid double QRS counting and T-wave oversensing. In this regard, a screening tool is used before implantation to confirm patients’ eligibility to the S-ICD by analysing the surface electrocardiogram (ECG) signals in both supine and standing positions (see Figure 1).
The implantation procedure is basically guided by anatomical landmarks with the option of fluoroscopy check to confirm optimal shock vector crossing the heart silhouette. At the end of the procedure, VF is induced using 50 Hz to assess correct detection and defibrillation (at 65 J) of the arrhythmia. Unlike the TV-ICD, the defibrillation test is still mandatory in the S-ICD since defibrillation threshold may be more dependent on the system positioning with fewer available long-term follow-up data.
Sensing the Subcutaneous Signals and Tachyarrhythmia Detection
Three sensing vectors are available in the S-ICD (see Figure 1); primary: sensing from the proximal electrode ring on the subcutaneous lead to the active surface of the PG; secondary: sensing from the distal sensing electrode ring on the subcutaneous lead to the active surface of the PG; and alternate: sensing from the distal sensing electrode ring to the proximal sensing electrode ring on the subcutaneous lead. Based on signal/noise and QRS/T ratios, the system automatically selects the best sensing vector to provide appropriate detection.
Automatic analysis of sensed signals basically includes three consecutive phases to avoid inappropriate sensing. 1) Detection phase: the device uses a detection threshold that is automatically adjusted continuously using amplitudes of recently detected events. 2) Certification phase during which the system excludes suspected events such as noise/artifacts, double QRS counting or T wave oversensing. 3) Decision phase: after excluding suspected events, only certified events are continuously analysed to calculate a running four R–R interval average, which is the indicator of heart rate.
In the shock zone (programmable between 170 and 250 bpm), rate is the only criterion used to determine if a rhythm will be treated with a shock. On the other hand, the optional conditional shock zone (programmable between 170 and 240 bpm) has additional discriminators used to differentiate supraventricular from ventricular tachyarrhythmias avoiding inappropriate therapies of the former. The discrimination algorithm in the conditional shock zone is based on the following sequential analysis: 1) correlation waveform analysis of each tachycardia beat with the stored baseline template. More than 50 % of correlation is considered normal activity suggesting supraventricular tachyarrhythmia; 2) beat-to-beat analysis, which considers polymorphic relationship as ventricular tachyarrhythmia, while in the case of monomorphic relationship the algorithm continues to the next analysis step; (3) QRS width analysis that indicates ventricular tachycardia if the QRS complex is wider than the baseline QRS template.
When 18 out of 24 consecutive tachy beats exceed the pre-determined therapy zone the device charges its capacitor to deliver an 80-J (non- programmable) biphasic shock, with shock polarity being automatically inverted after an initial unsuccessful therapy. Spontaneous termination of the arrhythmia during capacitor charging leads to shock abortion in order to avoid treating non-sustained episodes. The system is capable of delivering only 30 seconds of post-shock transcutaneous pacing if bradycardia is detected. Telemetric control of the S-ICD is provided by a small-size portable programmer allowing review and/or programming of all device diagnostics/settings including battery status, shock impedance, therapy and post-shock pacing activation, conditional shock VT and shock VF detection zones and stored arrhythmic events.
Commercial Phase and Clinical Data Regarding the S-ICD Performance
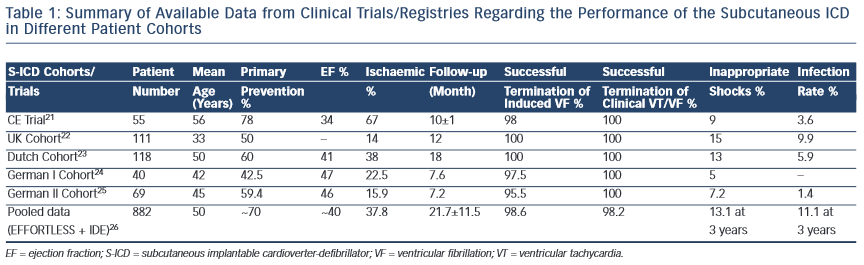
After preclinical and clinical research, the S-ICD received CE mark and US Food and Drug Administration (FDA) approval in June 2009 and September 2012, respectively. Several clinical studies have reported on the safety and efficacy of the S-ICD in primary and secondary prevention of SCD, and in different cardiac etiologies (see Table 1).21–26
The most available data on the S-ICD performance were obtained through pooled data from two large registries: IDE (S-ICD System IDE Clinical investigation) and EFFORTLESS (Boston Scientific Post Market S-ICD Registry).26 Data from these registries were recently published analysing S-ICD performance in 882 patients followed for 21.7±11.5 months.
The incidence of appropriate shock was 5.3 % over 1 year in this pooled cohort of S-ICD patients, which is relatively low compared with what has been reported with the conventional ICD. The annual incidence of appropriate ICD therapy (shock or antitachycardia pacing [ATP]) varied widely in the TV-ICD trials ranging from about 5 % (SCD- Heft) to 21 % (AVID).1,27 This is mainly due to different inclusion criteria enrolling patients with secondary prevention (Antiarrhythmics Versus Implantable Defibrillators Trial [AVID]), non-sustained/inducible VT (Multicentre Unsustained Tachycardia Trial [MUSTT], Amiodarone Versus Implantable Cardioverter-defibrillator Randomised Trial [AMIOVIRT]) or severely reduced EF (<30 %; Multicentre Automatic Defibrillator Implantation Trial II [MADIT II]).3,4,27 The pooled S-ICD cohort included relatively younger patients (~50 years), mainly with primary prevention indication (~70 %), and more than 20 % prevalence of channelopathies, electrical and genetic heart disease.26
Another important factor to be considered is the setting of ICD therapies. The incidence of appropriate therapy in the S-ICD was somewhat similar to that in the Sudden Cardiac Death in Heart Failure Trial (SCD-Heft) trial.1 The latter trial was conducted in patients with primary prevention indication (ejection fraction [EF] <35 %) and the ICD therapy consisted of a single-lead, single-shock zone (>187 bpm) device with no ATP being programmed.1 This ICD setting mimics the S-ICD design, which aims to treat fast ventricular tachyarrhythmias and has no ATP capability. Indeed, in the pooled S-ICD data the lowest-therapy zone was usually set at 200 bpm, with dual shock zone being activated in about 80 % of patients.26 The Multicentre Automatic Defibrillator Implantation Trial-Reduce Inappropriate Therapy (MADIT- RIT) trial addressed how the ICD programming may affect the incidence of appropriate therapies.28 Appropriate ATP therapy occurred in 22 % of patients in the conventional programming arm compared with 8 % and 4 % in the high rate and delayed therapy arms, respectively. However, there was no difference in appropriate shock (5 % with conventional programming or high rate programming, and 4 % with delayed therapy over 1.4 years). This study highlights the fact that conventional ‘aggressive’ ICD programming may overestimate the real incidence of appropriate therapies by treating potentially non-sustained episodes ‘unnecessary therapies’.
Notably, in the S-ICD pooled data about 36 % of detected VT/VF episodes were self-terminated, reflecting a deliberate time-delay strategy and a longer time-to-therapy (~20 seconds).26
About 90 % of spontaneous VT/VF events were terminated with first shock, and 98.2 % were terminated within the five available shocks. The estimated 3-year device-related complications and all- cause mortality were 11.1 % and 4.7 %, respectively. Importantly, no lead failures nor S-ICD related endocarditis/bacteraemia were reported. Inappropriate shock rate was 13.1 % at 3 years, mostly secondary to T-wave oversensing (about 40 % of inappropriate therapies). In patients with dual-zone programming at the index procedure, the incidence of inappropriate shocks at 3 years was significantly lower (11.7 %) compared with those with single-zone programming (20.5 %). These data further confirm the START (Subcutaneous versus Transvenous Arrhythmia Recognition Testing) study results, which highlighted the accuracy of S-ICD detection algorithms to discriminate supraventricular from ventricular tachyarrhythmia.29
Although current guidelines30 do not include the S-ICD as an alternative therapy, the available clinical data outline the potential benefit of this technology in selected patients at high risk of SCD. However, huge amounts of clinical data are available regarding the safety and efficacy of the TV-ICD while long-term data concerning the S-ICD performance are still lacking and need further research. The Prospective, Randomised Comparison of Subcutaneous and Transvenous Implantable Cardioverter-defibrillator Therapy (PRAETORIAN) trial, which is a randomised, controlled, multicentre, prospective double-arm trial, including 700 patients randomised (1:1) to subcutaneous versus transvenous ICD therapy, should provide further data in terms of safety and efficacy comparing both technologies.31 The S-ICD System Post Approval Study (ClinicalTrials.gov identifier: NCT01736618) is a non-randomised registry targeting to enroll more than 1,600 subjects at up to 150 investigational sites to analyse the long-term (5-year) performance of the S-ICD system. Another ongoing clinical trial is the Understanding Outcomes With the EMBLEM S-ICD in Primary Prevention Patients With Low Ejection Fraction (UNTOUCHED) Study (ClinicalTrials.gov Identifier: NCT02433379), which will assess the 18-month incidence of all-cause shocks in patients implanted with the new-generation S-ICD for primary prevention of SCD. Devices are to be programmed with zone cutoffs at 200 bpm and 250 bmp in order to mimic the programming settings for TV-ICDs in the MADIT-RIT study.28
S-ICD – In Which Patients?
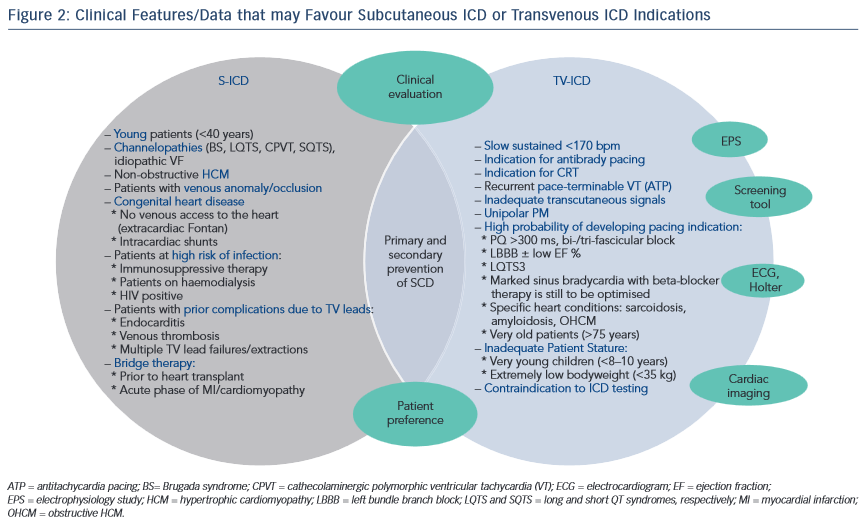
In general terms, all patients with an ICD indication may find in the S-ICD an alternative therapeutic option if pacing is not required. Those who have pacing indications (i.e. antibrady, cardiac resynchronisation therapy [CRT] or ATP) are not eligible for the S-ICD. In addition to the general ICD population, specific patient cohorts seem to benefit more from an S-ICD. TV-ICDs are often problematic in young patients (i.e. <40 years) who have long life expectancy and an active lifestyle making them more prone to lead failures, thus requiring multiple lead revision/ extraction procedures. Particularly, young patients with electrical heart disease/channelopathies (e.g. long and short QT syndromes, catecholaminergic polymorphic VT, Brugada syndrome and idiopathic VF) may benefit more from an S-ICD since their index arrhythmia is usually VF or polymorphic VT unresponsive to ATP. Moreover, patients who had already experienced serious complications related to TV leads (endocarditis, lead failures and inappropriate shocks, venous occlusion/thrombosis) should be considered for S-ICD if pacing is not indicated. Due to its subcutaneous nature, the risk of serious infections (bacteraemia, endocarditis) is extremely low with the S-ICD, making it a favoured option in patients at high risk of infection, such as those on immunosuppressive therapy, HIV patients or patients on haemodialysis who also often have vascular access concerns. Furthermore, TV-ICDs are problematic or unfeasible in some patients with congenital heart diseases (intracardiac shunts, venous occlusion/anomaly, extracardiac Fontan), in whom the S-ICD technology may offer a practical alternative to the more complex surgical approach.32,33 Considering the simplicity of its implantation and extraction, the S-ICD may also play a role in patients with a temporary risk for ventricular arrhythmias, such as prior to cardiac transplantation, the acute phase post-myocardial infarction/ myocarditis or recent onset of dilated cardiomyopathy. Additionally, some women may prefer the S-ICD for cosmetic factors as the PG pocket could be performed and hidden under the left breast.
On the other hand, caution should be taken before considering the S-ICD in patients with a higher probability of developing pacing indications in the near future. These may include patients with significant brady-arrhythmias/conduction defects, and specific cardiomyopathies known to be associated with monomorphic VT which may be pace-terminable. Detailed discussion with patients and families is required to address this issue with a potential future shift to a conventional TV-ICD. The role of an electrophysiology study to evaluate the propensity to a pace-terminable VT, favouring TV-ICD, is still to be determined. Figure 2 highlights the clinical features that may help to select the appropriate ICD technology for each patient.
How to Reduce Inappropriate Shocks in S-ICD Patients
Inappropriate shocks are a major concern in all ICD systems and are associated with increased mortality and reduced quality of life.34 Regarding the S-ICD a few considerations should be followed in order to minimise these undesired therapies:
- Prior to implantation: patient screening to ensure adequate transcutaneous signals (pre-operating screening tool) and to exclude those with high probability of double QRS counting/T- wave oversensing, which presents the most common cause of inappropriate shocks in S-ICD patients. In one study about 8 % of S-ICD candidates had inadequate transcutaneous signals that were mostly predicted by negative T-waves in lead I and the inferior leads of the surface ECG.35
- After implantation, sensing optimisation to select the best sensing vector (supine/standing positions).
- Dual zone programming is preferred (e.g.conditional shock zone 190– 220 bpm, shock zone >220 bpm) as it was significantly associated with inappropriate shocks reduction in the EFFORTLESS registry.26
- Exercise test may be helpful to evaluate the occurrence of myopotential oversensing/functional bundle branch block during exercise, with the possibility of selecting the best sensing vector and/or to update the QRS template.36
Developments in detection algorithms, such as the T-wave oversensing (TWOS) algorithm, may further optimise S-ICD functioning and reduce the incidence of inappropriate therapies.37
The EMBLEM S-ICD – A New Generation and Future Expectations
Since March 2015, a new generation of the S-ICD device, EMBLEM, has been developed and launched commercially by Boston Scientific. The EMBLEM S-ICD has several favourable features (see Figure 3) compared with the first S-ICD generation (SQ-RX 1010, Cameron Health):
- Smaller PG size: with a substantial reduction in volume (i15 %, from ~70 to ~60 cc), thickness (3mm less, i20 % from 15.7 to 12.7 mm) and weight (i10 %, from 145 to 130 g).
- Device shape: the edges of the new PG are more rounded and smoother to make its placement in the pocket easier and more comfortable. Furthermore, the header is centred to facilitate the subcutaneous electrode-wrap. These modified physical features of the new generation might be important to reduce patients’ discomfort, and importantly, the mechanical stress/skin erosions and thus pocket complications, including haematoma and infections.
- AnotherimportantfeatureoftheEMBLEMS-ICDisthelongerexpected battery longevity (h40 % from 5.1 to 7.3 years). Battery longevity is the determinant factor to reduce replacement interventions that are associated with significant costs and infection risk.
- The new device generation is compatible with remote monitoring (LATTITUDE). This feature might be particularly useful in the S-ICD since there are only a few parameters to be controlled/ programmed allowing the majority of patients to be followed, unless a thorough clinical assessment is required.
- Other technical improvements including the ability to store and print VF induction (defibrillation testing), and Bluetooth pairing/transfer.
Even after 15 years of continuous research and studies, the S-ICD technology is still evolving and the EMBLEM S-ICD represents one of its most recent advances. However, future research and design improvements are still required to address various aspects. For example, a paediatric model of the S-ICD to be used in small children (e.g., < 8 years, < 30 kg) may be an alternative option in the future. At least theoretically, subcutaneous defibrillation would require lower energies in these subjects due to their small cardiac mass and trans- thoracic impedance, and thus, a smaller paediatric model of the S-ICD with lower-energy might be feasible. Ongoing improvements in detection algorithms, as with the recent algorithm to avoid TWOS, should further improve S-ICD performance and reduce inappropriate therapies. The possibility of simultaneously analysing three, rather than one, available sensing vectors to define the rhythm status might be an advantageous feature to be considered and developed in the future. Finally, the integration of the S-ICD system with leadless pacing, if proved feasible, could play an important role in defibrillation technology enabling the expansion of the less invasive S-ICD therapy to a larger cohort of ICD population.
Clinical Perspective
- The S-ICD system represents a viable alternative to TV-ICD for primary and secondary prevention of SCD unless pacing is required.
- Its implantation is less invasive, does not require fluoroscopy and avoids the shortcomings related to TV leads.
- Growing clinical data are available regarding the safety and efficacy of this defibrillation therapy (EFFORTLESS registry).
- Careful patients selection and efforts to minimise inappropriate shocks are essential to optimise the clinical outcome of the S-ICD.
- Those who may particularly benefit from this technology are young patients, those with channelopathies or patients who have already experienced TV lead complications.
- The second-generation S-ICD (EMBLEM) has several favoured features including a smaller PG, longer longevity and remote-monitoring compatibility.
- Further innovations in the S-ICD system, detection algorithms and potential integration with leadless pacing in the future, may make this therapy suitable for a larger cohort of patients at high risk of SCD.