Over the past decade, the role of the gut microbiota in the pathogenesis of cardiovascular diseases has gained significant attention. Beyond its influence in shaping gut metabolism, these microbes also affect non-gut organs, which in turn has wide clinical and therapeutic implications.
The Gut Microbiota
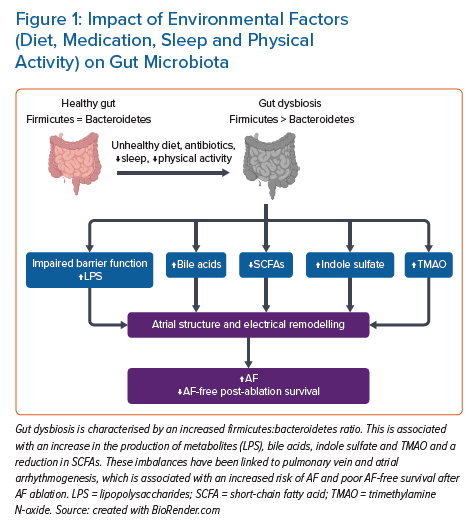
The term ‘gut microbiota’ was coined by Joshua Lederberg in 2001 and refers to the dynamic ecosystem of commensal symbiotic and pathogenic microorganisms that reside in the human gut, with their collective genome referred to as the ‘gut microbiome’.1,2 The adult gut microbiota is diverse and mainly consists of the phyla Bacteroidetes and Firmicutes. The gut microbiota is acquired at the time of birth and it subsequently evolves over time in response to diet, medications and disease.3 Its interaction with the food we ingest produces bioactive metabolites, referred to as the ‘metabolome’, which play a vital role in the maturation and regulation of the immune system of the host.4 Disruption of the gut microbiota, that is, ‘gut dysbiosis’, has been linked to a number of host cardiovascular diseases.5 Studies on the diversity of the human microbiome and differences between healthy and diseased individuals started in the 1680s with Antonie van Leewenhoek.6 However, over the last decade there have been significant advances in the tools to assess the gut microbiome, which have shed light on its link with cardiovascular diseases including AF (Figure 1).
How to Assess the Gut Microbiota
Conventional microbiology methods of inspecting bacteria compositions include using selective in vitro culture: by varying the nutrients available in culture media and the conditions (e.g. aerobic/anaerobic, temperature), the input mixture of bacteria could be segregated by their growth requirements before further investigation of isolated bacterial colonies such as morphological and biochemical analysis. However, generally only a minor portion of species from the gut can be cultured; they are mostly obligate anaerobes that are not viable from stool samples.7 In contrast, molecular techniques relying on DNA sequence differences do not require cultured cells, and can provide a more complete picture of the gut microbiome. Polymerase chain reaction amplification with species-specific DNA primers can identify the presence of a clinically important species (such as a suspected pathogen). To efficiently answer microbial ecology questions, bacterial 16S sequencing provided a major contribution: this method became possible with technologies for parallel (‘next-generation’) DNA sequencing, in this case for reading the DNA sequences from many amplified copies of the bacterial 16S (ribosomal) rRNA gene, which contains hypervariable regions useful for distinguishing bacterial species.8 In practice, 16S experiments may typically be targeted to one or two of the variable regions (e.g. V3–V4) and provide sufficient distinguishing sequence information to reliably classify bacteria down to the order or genus taxonomic levels; full 16S sequencing (including all nine variable regions) substantially improves classification confidence to the species level.9 A similar approach was developed for fungi, targeting the internal transcribed spacer sequences. Shotgun metagenomics is a more comprehensive approach, in which all of the microbial DNA is fragmented and aligned to genomic databases (species and strain-specific reference genomes) to provide information about microbial composition (including bacteria, fungi, viruses, protists) as well as insights into enrichment of functional genes groupings, such as genes encoding enzymes for the breakdown of particular substrates.10
The metabolites produced by bacteria are included in the circulating metabolome, which can be investigated by biochemical profiling methods from biofluids including serum or plasma samples.11 The gas chromatography-coupled mass spectrometry (GC-MS) technique involves chemically derivatising samples to make them volatile, and separable in gas phase when a temperature ramp is applied; separated compounds are identified by MS, which gives the precise molecular mass of compound fragments that are matched.12 Complementary methods include liquid chromatography MS (LC-MS), which is a similar approach with more coverage of detectable metabolites; and nuclear magnetic resonance spectroscopy offers advantages in measuring some classes of metabolites (such as lipoproteins) and inorganic metabolites or ions.13
Gut Microbiota and AF
AF, the most common sustained heart rhythm disorder in humans, affects 33.5 million people globally.14 Both the pulmonary vein and atrial substrate play an important role in the initiation and perpetuation of AF. There is extensive evidence of atrial and pulmonary vein remodelling in different population types with AF.15,16 However, the pathophysiology of such remodelling remains a point of considerable ongoing debate. It is proposed that inflammasome activation has a casual role in the aetiology of AF.17 The inflammasome, an intracellular multiprotein complex that functions as a molecular platform to activate the cysteine protease caspase 1, controls the production of proinflammatory cytokines such as interleukin (IL)-1β and IL-18.18 The NACHT-, LRR- and pyrin domain-containing 3 inflammasome (NLRP3) is the main inflammasome linked to the pathology and progression of cardiovascular diseases.19 NLRP3 inflammasome activity is increased in atrial cardiomyocytes of patients with AF.20 This inflammasome activation is associated with atrial hypertrophy, fibrosis, shortening of refractory periods and AF susceptibility.21 In addition to the role of inflammation, changes to autonomic nervous system activity can create a substrate that maintains AF.22
Patients with AF often have the same modifiable risk factors for coronary artery disease, including obesity, hypertension and diabetes, all of which are substantially linked to dietary habits. Given the current knowledge on the role of the gut microbiota on hypertension, obesity and atherosclerosis, its effect on the pathophysiology of AF is becoming a focus for research.23
Gut Microbiota Changes are Associated with AF
A number of observational and small cohort studies have identified distinct gut microbiota features in AF individuals. Dramatic alterations in the microbial diversity with a specific perturbation of gut microbiota composition has been shown in AF compared with control individuals.24 Overgrowth of Ruminococcus, Streptococcus and Enterococcus, as well as reduction of Faecalibacterium, Alistipes, Oscillibacter and Bilophila were detected in patients with AF. A gut microbial function imbalance and correlated metabolic pattern changes were observed with AF in both faecal and serum samples. Moreover, Tabata et al. showed that such alterations are related to dietary habits when 34 AF patients were compared with 66 controls.25 A depletion in Enterobacter species and an enrichment in Parabacteroides, Lachnoclostridium, Streptococcus and Alistipes was observed in AF patients. This corresponded to an increase in intake of n-3 polyunsaturated fatty acids and eicosadienoic acid in AF individuals. In addition, gut dysbiosis was associated with progression and duration of AF. A recent study by Zuo et al. examined the microbial diversity and metabolite composition in a cohort of patients with paroxysmal AF, persistent AF and individuals without AF and confirmed prior observations that AF patients had significant microbial diversity compared with individuals without AF.26 Additionally, certain bacteria were differently enriched at different AF durations (decreased abundance of Butyricicoccus and Paraprevotella; and increased abundance of Blautia, Dorea and Coprococcus in persistent AF patients), suggesting that persistent AF may be related to the imbalance of gut microbiota. Interestingly, changes to the enrichment of intestinal microbes were observed after AF ablation compared with before ablation, with enrichment in beneficial bacteria and reduction in pathogenic bacteria and a corresponding change in metabolite levels.27 However, the causal relationship between AF and gut microbiota remains unclear. Certainly, Xu et al. showed in a bidirectional Mendelian randomisation analysis using a Chinese cohort that AF itself can induce the abundance of specific gut microbes.28
Potential Mechanisms Linking Gut Dysbiosis with Development and Progression of AF
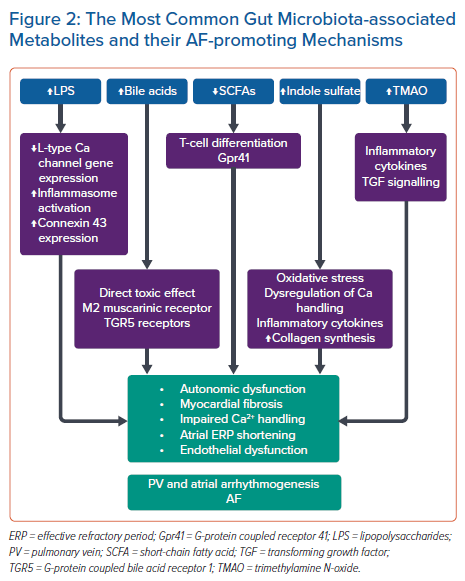
In this section we review the current available evidence that supports the role of the gut microbiota in the pathogenesis of AF. The gut microbiota’s interaction with our diet produces a number of metabolites that form the foundation for the human gut–heart axis through a number of AF-promoting mechanisms (Figure 2).
Trimethylamine N-oxide
Trimethylamine N-oxide (TMAO) is a gut microbial-derived metabolite that has been recently suggested to play a role in the pathogenesis of AF. TMAO is derived from dietary choline or L-carnitine through gut microbiota, which transforms choline to trimethylamine, a gas absorbed into the circulation, which is further converted into TMAO by flavin-containing monooxygenases in the liver.29 TMAO levels in the blood can be measured using LC-MS, with a fasting plasma normal range in healthy individuals reported to have a median level of 3.45 µM (IQR: 2.25–5.79 µM).30
Several cohort and systematic review studies have linked TMAO with hypertension, obesity, heart failure and coronary artery disease.24,31–35 Putative mechanisms included its impact on cholesterol metabolism, endothelial dysfunction and platelet activity, which may enhance the development of atherosclerosis.36 However, the link between AF and TMAO remains a topic of ongoing debate. Given that AF shares similar risk factors to coronary artery disease, it is currently unclear whether TMAO in AF is causative or a bystander in AF.
In the Western Norway Coronary Angiography Cohort (WECAC), an AF incidence of 10.9% during a 7-year follow-up correlated with higher baseline TMAO levels.37 This prospective association was independent of traditional AF risk factors (age, gender, smoking, hypertension, BMI and diabetes). However, in contrast, Büttner et al. noted similar TMAO plasma levels between AF and non-AF individuals when individuals with coronary artery disease were excluded.38 Further support of a link between TMAO and AF was demonstrated by Nguyen et al., who detected a significant association between TMAO levels and AF clinical phenotype.39 In a cohort of 78 patients, TMAO levels were significantly higher in 22 patients with persistent AF compared with 56 patients with paroxysmal AF. However, that study did not adjust for the traditional risk factors of AF that are known to cause AF and which are more prevalent in persistent AF than paroxysmal AF. Contrary to the findings of this association between AF and TMAO, a number of studies did not find such an association. In a cohort of 45 AF patients and 20 healthy matched controls, baseline TMAO levels were similar and did not differ between paroxysmal or persistent AF or healthy controls.26 In a follow-up analysis of the PREDIMED study, no association between AF and TMAO levels was found.40 However, they did find an association between baseline plasma choline, betaine and dimethylglycine levels and the risk of incident AF and heart failure.
A number of animal studies have highlighted a range of possible mechanisms that link TMAO with AF. These mechanisms include cardiac autonomic nervous system activation, inflammation, oxidative stress, endothelial dysfunction and myocardial fibrosis. In a recent study using a canine model, Yu et al. demonstrated activation of inflammatory pathways by TMAO, leading to increased autonomic activity and AF induction.41 Specifically, TMAO was injected into four major anterior right ganglionated plexi, resulting in acute electrical remodelling compared with saline controls. This was also associated with a significant increase in the expression of the proinflammatory factors interleukin (IL)-1β, IL-6 and tumour necrosis factor-α.29 The effect of TMAO on atrial tissue in a cohort of rabbit hearts has also recently been investigated.42 When compared with normal saline, TMAO promoted proliferation and migration of atrial fibroblasts by activating the β-catenin pathway. Neonatal rat cardiomyocytes exposed to TMAO showed an increase in mRNA and protein levels of hypertrophy markers such as atrial natriuretic peptide and beta-myosin heavy chain, along with more fibrosis via unregulated transforming growth factor signalling pathways.43 TMAO levels were found to increase with age; and treatment of a mouse model with TMAO led to induction or acceleration of vascular age, as measured by an upregulation of senescence markers, including senescence-associated B-galactosidase.44
Short-chain Fatty Acids
Short-chain fatty acids (SCFAs) are metabolites produced by gut microbiota ingestion of dietary fibre. Acetate, propionate and butyrate are the most important and biologically active, accounting for 95% of SCFAs.45 SCFAs have a beneficial immunomodulatory effect on the host through a range of mechanisms.46–48 Zhang et al. recently studied the differences in the microbial genes involved in SCFA-related synthesis in a Chinese cohort.49 AF patients, compared with non-AF patients, had a marked reduction in their SCFA-producing microbes and genes. However, the evidence that links SCFAs to AF is currently limited and most available evidence links it with the progression of traditional AF risk factors such as hypertension, obesity and atherosclerosis. In a mouse model, Kasahara et al. demonstrated that colonising mice gut with butyrate-producing bacteria species (Roseburia sp.) was associated with reduction in endotoxaemia and atherosclerosis development.50 A high-fibre diet and acetate supplementation was associated with reduction in blood pressure and downregulation of the genes associated with the development of cardiac hypertrophy, cardiorenal fibrosis and inflammation.51
Primary and Secondary Bile Acids
Previously considered as mere ‘detergent’ molecules responsible for the absorption of dietary fats and lipid-soluble vitamins, there is now compounding evidence that bile acids have circulating signalling molecules that have the ability to regulate cell biology, metabolism and function of various extrahepatic organs.52,53 The primary biliary acids cholic acid and chenodeoxycholic acid are subsequently conjugated in the liver with the amino acids taurine or glycine. Following secretion into the gut during food digestion, intestinal microbiota causes their deconjugation and dihydroxylation via 7-alpha dehydroxylase, to form the secondary bile acids deoxycholic acid and lithocholic acid, respectively.52,53 These bile acids are reabsorbed back into the liver via the portal veins. This enterohepatic circulation is disrupted during liver disease, leading to spillage of these metabolites into the systemic circulation, leading in turn to end organ dysfunction. Wang et al. demonstrated that serum concentration of chenodeoxycholic acid was significantly higher in AF than in control patients.54 In addition, they noted a positive association with left atrial size and low-voltage areas on electroanatomic mapping. The secondary bile acids glycolithocholate sulfate and glycocholenate sulfate have been shown to have a significant association with the risk of AF in an African-American patient cohort.55 The possible mechanisms that imply a bile acids–AF relationship include the impact of bile acids on the structure of the cardiomyocyte cell membrane by causing direct damage to cardiac ion channels and affecting Ca2+ signalling; and the intracellular effect of bile acids on pathways that regulate Ca2+ homeostasis and signalling through membrane receptors such as muscarinic receptors or the bile acid receptor TGR5 (G-protein coupled bile acid receptor 1).56–58
Lipopolysaccharides
Lipopolysaccharide (LPS) is an endotoxin that serves as a major structural component of the outer layer of Gram-negative bacteria.59 Translocation of LPS into the systemic circulation occurs as a consequence of increased gut permeability and has been linked to hypertension and atherosclerosis in human studies.34 In AF, animal studies have demonstrated the potential impact of LPS on atrial arrhythmogenesis.60,61 In an experiment involving the intraperitoneal injection of LPS into rats, this was associated with downregulation of L-type calcium channel gene expression, atrial effective refractory period shortening, and an unstable atrial response to programmed electrical stimulation.60 In addition, Chen et al. showed that LPS treatment in a canine model led to activation of nuclear factor kB (NF-kB), increase in plasma and atrial concentrations of proinflammatory markers, and increasing connexion 43 expression and lateralisation, which led to atrial effective refractory period shortening and inducible AF.61 Recently, preclinical studies have shown that both ageing and a high-fat diet can alter the gut microbiota and promote LPS production.62,63 When a group of young rats underwent transplantation of faecal microbiota from an older rats group with increased AF susceptibility, the transplanted young rats cohort had a dramatic increase in circulating LPS concentration, glucose, upregulation of NLRP3 inflammasome, atrial fibrosis, and subsequently inducible AF.63 Kong et al. showed that transplantation of faecal microbiota from mice that had been fed a high-fat diet to a group of mice on a normal diet led to a significance increase in LPS concentrations, increased inflammatory cytokines, upregulation of NF-kB and NLRP3 inflammasome activity, and AF inducibility.62 In human studies, microbes involved in LPS synthesis were enriched in the gut of AF patients, and this was associated with an upregulation of LPS synthesis by encoding the LPS-enzymatic biosynthesis gene.64 Moreover, circulating LPS levels were positively associated with major adverse cardiovascular events and platelet activation in AF patients, and were negatively affected by high adherence to a Mediterranean diet rich in fruits and legumes.65
Tryptophan and Indole Derivatives
Tryptophan is an essential amino acid for maintaining health and homeostasis. When dietary tryptophan is metabolised into indole by intestinal microbiota and absorbed into the bloodstream, it would then be converted to indoxyl sulfate in the liver. Indoxyl sulfate is a uremic toxin associated with cardiovascular adverse effects in patients with chronic kidney disease as a result of the impaired systemic clearance of this metabolite.66 In a rabbit experimental model, it increased pulmonary vein and left atrial arrhythmogenesis and reduced sinoatrial pacemaker activity by causing oxidative stress and dysregulation of cardiomyocyte calcium handling.67 Moreover, injection of indole sulfate into a rat model led to stimulation of fibroblasts and cardiomyocytes, increased collagen synthesis, and myocyte hypertrophy in addition to an increase in proinflammatory cytokines.68 In human studies, elevated indoxyl levels at baseline were associated with an increased risk of AF recurrence after ablation.69 However, successful catheter ablation was associated with a decrease in indoxyl sulphate levels during follow-up, which was independently associated with an improvement in estimated glomerular filtration rate, suggesting that this would be a mechanism for indoxyl sulfate reduction rather than AF itself.70
Therapeutic Implications
Despite the current lack of human studies to support interventions aimed at modifying the gut microbiota in the prevention and management of AF, recent preclinical animal studies have highlighted the potential impact of gut microbiota modification on AF susceptibility.62,63 Zhang et al. showed that transplantation of young faecal microbiota into AF-susceptible aged rats led to a reduction in LPS concentration, suppression of NLRP3 activity, decreased atrial fibrosis and AF inducibility.63 Moreover, several studies have shown promise towards managing AF risk factors. Probiotics rich in Lactobacillus spp. and Bifidobacterium spp. have been shown to be effective in the management of AF risk factors such as atherosclerosis, dyslipidaemia and hypertension.71–73 Supplementation with SCFAs (propionate) is associated with improved insulin sensitivity and blood pressure control.74,75 Faecal microbial transplantation in humans has been associated with improved insulin sensitivity in patients with metabolic syndrome.76 Future studies are required to determine whether the aforementioned interventions have an impact on AF pathogenesis.
Future Directions
Although an extensive body of evidence is present to support differences in gut microbiota in AF patients compared with healthy controls, direct evidence linking gut-associated metabolites to AF susceptibility is still lacking. Large studies comparing individuals with and without gut dysbiosis and adjusted AF risk factors are needed.
In addition, although current interventions such as probiotics, faecal microbial transplantation and diet have shown promise in the management of AF risk factors, there is no clear evidence to support their role in the prevention or treatment of AF. Randomised clinical studies to study the impact of each component are required to answer that question.
Last, elevated blood concentrations of gut-associated metabolites have been associated with recurrence following AF catheter ablation in small observational studies. Large studies are required to determine whether using such metabolites would add to the current clinical recurrence prediction algorithms to assist in choosing patients who are likely to benefit from AF ablation.
Conclusion
With advancements in the molecular assessment of the gut microbiota, there is an emerging body of evidence to suggest that there is a link between gut dysbiosis and AF. Animal and human experimental studies are required to determine the mechanistic link between gut dysbiosis and arrhythmogenesis in AF. This would pave the way towards conducting clinical interventional studies to determine whether the gut dysbiosis is a modifiable AF risk factor.
Clinical Perspective
- Distinct alterations in the gut microbiota are present in AF patients.
- These alterations mediate the production of a number of metabolites that are proposed to influence the arrhythmogenesis of AF. However, the precise mechanistic link remains unknown.
- Future interventional studies are required to determine whether dietary and faecal transplant interventions have an impact on gut dysbiosis.