Remote monitoring (RM) of implanted cardiac devices to detect device-related issues and clinically relevant arrhythmias is well established and endorsed by international guidelines.1 Existing infrastructure and technology facilitate passive transmission of device-monitored health data from a patient’s own home to their healthcare provider, allowing for real-time diagnosis and management. In recent years, heart failure (HF) diagnostics have been added to the range of features available to clinicians. Despite good evidence of predicting acute HF episodes, results from initial clinical studies focused on reducing HF hospitalisations were conflicting; thus, enthusiasm slowed and guidelines remained largely unaltered.2–5 However, recent studies have produced promising results that, alongside radical changes to the delivery of care during the COVID-19 pandemic, have reignited interest in RM platforms.5,6 There has also been an observed shift in focus towards the applied utility of device diagnostics to augment clinical pathways rather than standalone diagnostic tools.7 This narrative review focuses on cardiac device HF RM tools currently available in the UK, provides an overview of the current research evidence and discusses the ways in which these RM tools have been integrated into clinical pathways to improve the care of device patients with HF.
Review Methodology
The aim of this narrative review was to summarise the available evidence based on critical appraisal of the literature. Articles included in the review were limited to published peer reviewed articles, national audit reports and policy documents available in English. This review may not include all available tools for HF device RM because a systematic review of the literature was not performed. The focus was on RM technology currently available in the UK market or undergoing active research.
Heart Failure and the Need for Remote Monitoring
Approximately 920,000 people in the UK have HF, with up to 80% of diagnoses being made in the hospital setting.8–10 Although therapies have rapidly advanced over the past 20 years, it is notoriously hard to achieve a chronic ‘state of remission’ for patients who commonly have multiple coexisting long-term health problems.8,11,12 HF is the primary diagnosis for more than 100,000 hospital admissions per year in the UK, with admissions projected to increase 50% by 2035.10 However, it is now widely accepted that many HF decompensations are predictable and can be managed in the ambulatory setting if caught early, avoiding the need for hospitalisation in many instances.13,14
For the most part, the mainstay of HF medical management is supporting patients with the highest tolerated dose of guideline-directed medical therapy (GDMT). Numerous studies have demonstrated that optimal GDMT promotes disease stability and prolongs life expectancy; yet despite this, decompensation episodes requiring escalated doses of diuretics, or modification of treatment for a new acute issue, are commonplace.15–19 For this reason, the National Institute of Health and Care Excellence (NICE) guidelines recommend people living with HF are monitored 6 monthly at a minimum, and this should include (as a minimum) a clinical assessment, medication review, renal function and patient education/support.15 Practices vary across the country but, for most, routine chronic disease management is divided between general practices, community HF services and outpatient secondary care HF teams. Unfortunately, acute HF is most commonly recognised in the hospital setting after emergency admission.9 For patients living with HF who develop symptoms to suggest decompensation, there is seldom direct access to rapid specialist care for early intervention.
The impact of the COVID-19 pandemic on people living with HF in the UK is difficult to assess with any degree of certainty.20 Research so far would suggest a drop in HF hospitalisations, but an excess number of HF deaths in the community. A recent retrospective review of cardiovascular deaths in the UK during the pandemic reported a 25% increase in HF deaths in care homes and hospices, and a 33% increase in HF deaths in people’s own homes.21,22 Studies around the world have reported a significant decline in HF hospitalisations since the beginning of the pandemic.23–25 Reports from multiple sites in London found a significant reduction in HF hospitalisations, but an increase in inpatient mortality.26,27 Most observers agree that during the COVID-19 pandemic HF patients were less likely to access medical services, managing symptoms at home where previously they would have sought advice. Service delivery for HF patients has also altered radically during periods of social restrictions, with telemedicine being an obvious solution for maintaining outpatient specialist services. For many healthcare professionals this simply involved switching to telephone consultations, without physically examining the patient or having clinical data to hand, such as blood results, unless there was an urgent need. As things return to normal, it remains unclear whether services will return 100% to the traditional format or whether some aspects of this new care delivery will stick.
Cardiac Devices and the Evolution of Remote Monitoring
Cardiac implantable electronic devices encompass a diverse range of therapies, including permanent pacemakers (PPM), ICDs and cardiac resynchronisation therapy (CRT) devices, with a pacemaker (CRTP) or with a defibrillator (CRTD). There are various indications for implantation but, for patients with HF, common indications for device-based therapies include: the use of ICDs for primary and secondary prevention of sudden cardiac death; and the use of CRT devices generally in patients with HF and broad QRS duration (left bundle branch block morphology), although guidelines vary slightly.1,28–30 ICDs are used for primary prevention in patients at high risk of sudden death (typically ejection fraction <35%, New York Heart Association [NYHA] class II–III despite 3 months optimal medical therapy, life expectancy >12 months) and for secondary prevention in case of ventricular arrhythmias. The use of CRTD versus CRTP is largely based on clinical judgement, taking into consideration indications for defibrillator therapy, the presence of fibrosis on cardiac MRI, the aetiology of HF and comorbidities.
CRT typically involves cannulation of the coronary sinus and delivery of a left ventricular pacing lead into a lateral branch, but can also be achieved by conduction system pacing, a technique that involves placement of the lead to capture the His or left bundle branch, in order to achieve what has been termed ‘physiological’ pacing. However, the impact of conduction system pacing on hard outcomes, including mortality benefit, over conventional CRT has not been demonstrated in large-scale randomised clinical studies.30
Historically, cardiac devices were programmed to transmit an audible alert in the case of device failure (e.g. extremes of low battery or lead integrity alert). As technology has improved, device capabilities have expanded to include auxiliary functions, using built-in sensors to collect health data to aid clinical decision making. Devices are typically able to store data for just over 1 year, until they are downloaded onto monitoring platforms from home, onto a mobile app or at an in-person evaluation. For home-based device transmissions, depending on the manufacturer, scheduled automated transmissions will ‘pull’ device data onto the RM platform if the patient is within the range of their home transmitter or mobile app on the day of the scheduled download. Between scheduled appointments, devices can be programmed to send automated downloads if certain criteria are met (i.e. arrhythmia or HF alert threshold crossed).
Remote Monitoring and Platforms
Although definitions may vary, RM has been defined as ‘automated transmission of data based on pre-specified alerts’, typically activated for potentially life-threatening arrhythmic events or abnormal device function, whereas ‘remote interrogation’ is defined as ‘routine, scheduled, remote device interrogation planned to mirror an in-office check’.31 Prior to the COVID-19 pandemic, although the British Heart Rhythm Society (BHRS) guidance acknowledged some non-CRT patients could be offered continuous remote follow-up provided set criteria were met, most continued with a minimum 12-monthly in-person interrogation and review. Pandemic restrictions have seen a radical shift by most hospitals towards sole, continuous RM across all device types and manufacturers, with BHRS guidelines altered in 2022 to reflect a move towards sole remote follow-up.31,32
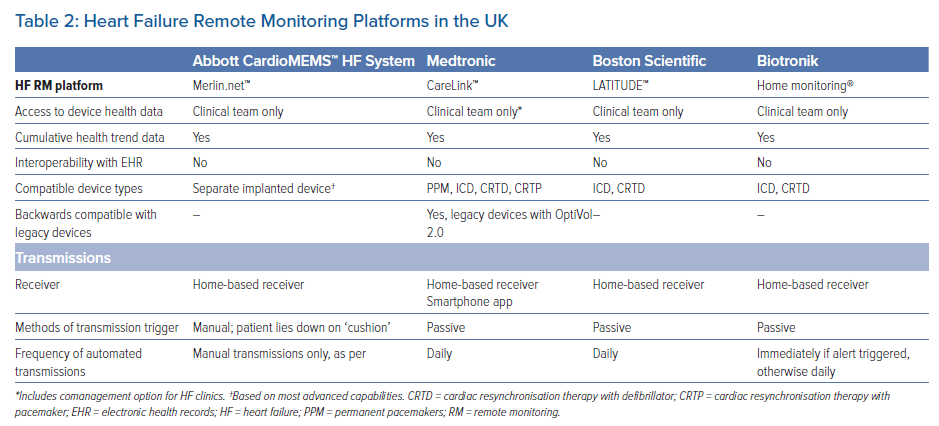
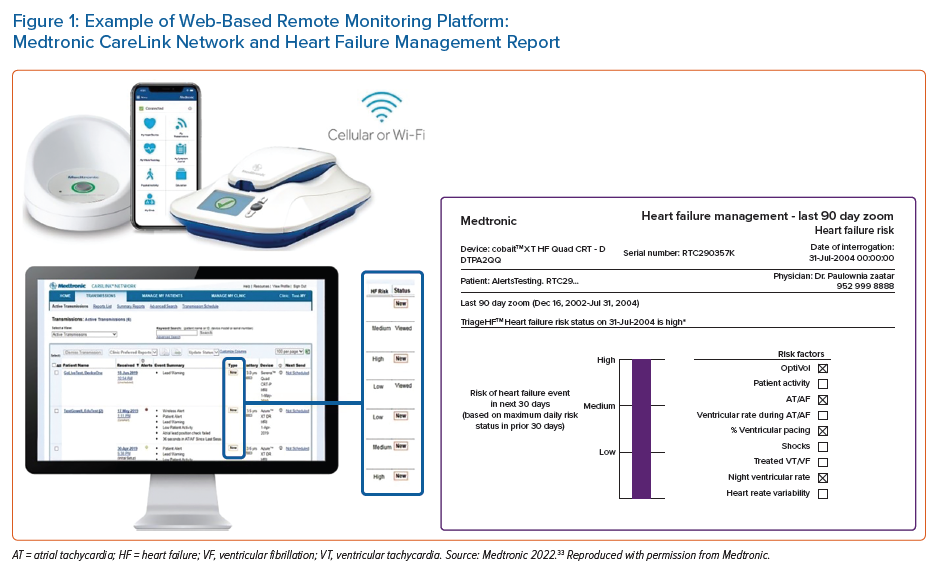
Data from device diagnostics is summarised and shared with healthcare providers via web-based RM platforms (Figure 1). Efforts have been made by manufacturers to separate health data from more technical aspects of device data to make the systems more user-friendly for non-technical users. All RM platforms share common characteristics, mainly graphical representation of trend data and most recent values. Details of individual parameters for the algorithm platforms are usually available, but historical data are commonly summarised. Although rapid progress has been made, RM platforms remain in their relative infancy. Interoperability with existing electronic health records is not available, and interactivity is not used (i.e. it tends to be a ‘read-only’ system). These issues are likely to be resolved as platforms increase in use; however, in the meantime, these issues may act as barriers to use beyond specialist providers. At present, no manufacturer offers patient access to device RM data.
Device Heart Failure Diagnostics
HF diagnostics can aid medium- to long-term clinical decision making at scheduled HF reviews, but the real focus of device diagnostics has been to create acute clinical early warning systems (i.e. to detect changes in physiological parameters before symptoms and signs of fulminant acute HF occur clinically). Sufficient lag time to implement preventative measures is essential for these systems to be clinically useful and a distinct advantage of device-HF alerts over traditional methods of disease monitoring outlined below.
Approximately 30% of all patients admitted to hospital with worsening HF will die within 1 year.34 Detecting an acute HF episode earlier in its clinical course could help prevent unnecessary hospitalisations and associated complications. Traditional measures of volume status, such as weight, are insensitive and only tend to become significant in the days leading up to HF hospitalisation.35,36 Symptoms such as ankle swelling and breathlessness are also late and non-specific features (Supplementary Material Figure 1).37 Research from monitoring technology embedded in cardiac devices has demonstrated that HF instability can be detected up to 6 weeks in advance of hospitalisation from sustained increases in filling pressure or pulmonary pressure.38–40
HF device diagnostics vary by manufacturer and model, but generally include parameters thought to predict acute HF events through single metrics, such as arrhythmia detection and measures of fluid status, and combinations of multiple parameters to create risk stratification scores.
Single Metrics
Arrhythmia Detection
Approximately 30% of acute HF episodes are precipitated by AF, 7% are precipitated by ventricular arrhythmias and 4% are precipitated by bradycardias.41 All CRT devices and most PPM and ICD devices will monitor for atrial arrhythmias and, depending on RM parameters and set-up, can transmit an alert to the healthcare provider in order to prompt clinical action. High-power devices can discriminate ventricular arrhythmias and store electrograms.42
Fluid Status
Fluid status can be measured by proxy (thoracic impedance) or by direct haemodynamic pressure monitoring (pulmonary artery pressure [PAP] or left atrial pressure [LAP]). Whereas thoracic impedance is readily available as an ancillary function of contemporary cardiac devices (CRT, ICD and PPM), invasive haemodynamic monitoring is performed by a separate PAP sensor system . At the time of writing, there are no commercially available LAP RM platforms available in the UK; the LAPTOP-HF trial of HeartPOD (St Jude Medical) was stopped early due to procedure-related complications.43,44 The only readily available PAP monitor in the UK is the Abbott CardioMEMS HF System (see below). Trial results from the SIRONA 2 Trial Heart Failure NYHA Class III, a safety and efficacy trial of the Cordella Pulmonary Artery Sensor System, a competitor PAP monitor, were recently published, but this system is not currently licensed for clinical use.45
Thoracic Impedance
Thoracic impedance quantifies pulmonary congestion by measuring electrical impedance through lung tissue (in the UK, available in the HeartLogic [Boston Scientific], OptiVol 2.0 [Medtronic], CorVue [Abbott] and Biotronik HeartInsight-compatible devices). There are many advantages to this system over haemodynamic pressure monitoring, primarily: it is easily embedded within existing device technology (mainly CRTs) and can thus be integrated with existing RM platforms; and measurements occur passively (i.e. the patient is not required to activate the sensor for the data to be collected). Nonetheless, despite significant advances in technology, device-derived measurements of thoracic impedance are generally considered to have poorer sensitivity and specificity for clinically significant pulmonary congestion than direct methods. In addition to congestion, impedance values can drop in response to respiratory or device-related infection, and as well as to pleural or pericardial effusions.46,47 Typically, daily impedance measurements are compared to a patient-specific reference impendence value, with an alert triggered by rising cumulative levels.48 Variability in the thresholds, along with manufacturer-specific processing procedures, may explain the mixed results in clinical studies.2
An example of thoracic impedance tracking is the OptiVol 2.0 (Medtronic), which can be viewed by clinicians as a single metric or as part of the TriageHF algorithm (see below). Data are collected from 34 days after implantation and displayed in graphical format as OptiVol fluid index and thoracic impedance. In an observational study of 33 patients, Yu et al. reported that impedance tended to drop approximately 15 days before the onset of worsening symptoms.49 The PARTNERS HF study reported that an OptiVol index ≥60 conferred a 2.7-fold risk of HF hospitalisation within 30 days, increasing to 3.3 and 3.9 if the threshold increased to ≥80 or ≥100, respectively.3 Although early studies were promising, research monitoring lone thoracic impedance with an audible alert did not show a reduction in HF hospitalisations (DOT HF and OptiLink HF).50,51 The extent to which the audible alert may have played a role in ‘sending’ patients to the hospital and ensuing unplanned hospitalisations remains unclear. Another example is the Abbott CorVue function (previously St. Jude’s Medical), which measures impendence across two vectors between the ventricular leads and pulse generator. Low impendence is sensed against a reference value in a similar way to OptiVol 2.0. The DEFEAT-PE study reported a sensitivity of 32.3% for ICDs and 32.4% for CRTDs for the detection of pulmonary congestion events; false positive rates ranged from 1.28 to 1.66 per patient-year.52
To summarise, the accuracy and reliability of thoracic impedance in isolation to detect HF decompensation has not been convincingly shown and is not recommended in clinical guidelines; however, in combination with other parameters, it has shown value in detecting clinically significant events.
Abbott CardioMEMS
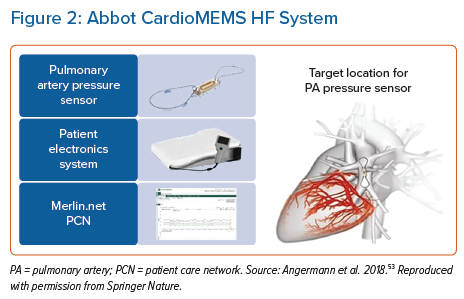
Haemodynamic pressure monitoring has proved more accurate for the detection of impending pulmonary congestion and has a class IIb(B) recommendation in the European Society of Cardiology (ESC) heart failure guidelines for symptomatic patients with HF with reduced ejection fraction.1 The Abbott CardioMEMS pulmonary artery pressure monitor is a small battery-free leadless coil and pressure sensor capacitor delivered into the pulmonary artery system, typically via a transfemoral delivery catheter (Figure 2).54 To record a measurement, patients are required to lie supine on a specialist receiver ‘pillow’ and activate via a remote control to send data to their healthcare provider via the Merlin.net patient care network. The device is currently indicated for patients with NYHA class II and III symptoms and either raised natriuretic peptide or an HF hospitalisation in the preceding year.55 Usability is limited to patients with a BMI ≤35 kg/m2 (if the BMI is higher, chest circumference must be <165 cm or additional measurements are required on angiography) and an estimated glomerular filtration rate >25 ml/min/1.73m2.56 Activating the sensor only takes a few minutes but does require an engaged patient with good cognition and dexterity to perform regularly. Correlation with PAP as measured by a Swan–Ganz catheter was strong (r2=0.96) in a pilot study.57
The 2007–09 CHAMPION study compared usual care versus RM based on daily CardioMEMS measurements for patients with NYHA class III and an HF hospitalisation within the past year. Default haemodynamic pressure monitoring targets were set to 10–25 mmHg for mean PAP, with clinicians advised to adjust diuretic therapy based on these targets.56 Over an average of 13 months follow-up, HF hospitalisations were reduced by 48% in the intervention arm (95% CI [0.40–0.69]; p<0.001).58,59 The follow-on 2018–21 GUIDE-HF study, which extended inclusion criteria to patients with NYHA class II–IV and those without a recent hospitalisation, was unfortunately hampered by the impact of the COVID-19 pandemic.60 That study recruited and implanted 1,000 patients between March 2018 and December 2019, with a 12-month follow-up. Although in the pre-COVID-19 follow-up period there was a significant difference in HF hospitalisations and visits between the RM and usual care groups (HR 0.81; 95% CI [0.66–1.00]; p=0.05), this effect was not sustained in the post-COVID-19 period (overall results: HR 0.88; 95% CI [0.74–1.05]; p=0.16).60 The authors concluded this neutral result was likely driven by a significant drop in the rate of hospitalisations in the control group during the COVID-19 pandemic (21%). The CardioMEMS HF System Post-Market Study (COAST) was also affected by the COVID-19 pandemic. However, the preliminary results from the pre-COVID follow-up period, published in December 2021, were very promising, with HF hospitalisation rates in the intervention group 82% lower than in the control group.5 Post-COVID-19 data are awaited (Figure 2).
Multiple Metrics
Multiple metrics can be visually displayed together based on scheduled transmissions or preprogrammed alerts. Most contemporary ICD or CRT devices monitor atrial and ventricular tachycardias, AF/atrial tachycardia burden, delivered shocks (defibrillators only), CRT pacing (CRTs only), heart rate, rhythm and PA, with additional metrics including heart rate variability, thoracic impedance, heart sounds, rapid shallow breathing, sleep incline, ventricular extrasystole and intracardiac electrogram.4,61–63 Alerts are based on single parameters, either by detecting a new event, a significant trend away from baseline or crossing a preset threshold. The IN-TIME study (Biotronik) is the only RM study in HF thus far to result in changes to ESC guidance (Class IIb recommendation for multiparametric monitoring in ICD patients with symptomatic HF and an ejection fraction ≤35% in the 2016 guidance; however, this was later dropped from the 2021 ESC guidelines citing the REM-HF study).14,64
IN-TIME Study (Biotronik)
The IN-TIME study, a 2014 randomised controlled trial of telemonitoring based on multiparametric device monitoring, recruited 716 HF patients across Australia, Europe and Israel.4 Patients were restricted to those with ICDs or CRT-D devices, chronic HF of NYHA class II–III, ejection fraction ≤35% and non-AF rhythm. Device data were transmitted daily to both a central site and local investigators, although only abnormal observations detected by the central site were forwarded on for action. Local investigators were given the discretion to act accordingly (telephone interview followed by clinical intervention as per standard practice). The most common clinical reason for forwarding on an observation was atrial tachyarrhythmia, followed by low CRT pacing. Approximately half of all forwarded observations triggered a telephone assessment (641/1,225) and only 8% (99/1,225) instigated action.4 At the 1-year follow-up, all outcome metrics were superior in the intervention compared with control group (clinical composite score worsened in 18.9% versus 27.2% [p=0.013]; mortality 3.0% versus 8.2% [p=0.004]).4
REM-HF Study
Multiple metrics from Medtronic, Boston-Scientific and Abbott devices were used to test the impact of RM in the management of HF (REM-HF study).2 The REM-HF study ran from 2011 to 2014 across nine sites in England, recruiting 1,650 patients with CRT or ICD devices capable of transmitting HF diagnostic data, with a minimum 2-year follow-up. The intervention arm received device-based RM care based on visual interpretation of trended health data, transmitted weekly. One healthcare professional per site was allocated to review downloads and act upon received data. Although there was a procedural handbook provided to sites to guide responses to device data, healthcare professionals were given the freedom to manage patients as clinically appropriate based on best-practice guidelines. The study ran as a time-to-event analysis, with no significant differences between the intervention and control groups in the primary outcome (all-cause death or cardiovascular hospitalisation; HR 1.01, 95% CI [0.87–1.18], p=0.87) or any of the secondary outcomes (cardiovascular/non-cardiovascular death/hospitalisation).2 During the study follow-up, 79,325 downloads were reviewed, with action taken at least once for 73% of patients during follow-up.2 Data overload and excessive review of ‘normal’ data were cited as undesirable unintended consequences of the protocolised weekly downloads. Following the publication of REM-HF, Brahmbhatt and Cowie reflected on the challenge of streamlining data to ‘provide signal rather than just noise’.65
REM-HF, the only cross-manufacturer randomised trial of RM in HF, is often cited as a reason to reject the utility of device HF RM, and appeared to be the main driver for multiparametric monitoring recommendations being dropped from the ESC guidelines. However, it is important to note that the results of REM-HF, although neutral, played a critical role in shaping the current landscape of RM, and informing best practice. There have been significant advances in device HF monitoring technology since 2011–14 when REM-HF was undertaken. First, device manufacturers (Medtronic, Boston Scientific and Biotronik) have moved away from using single metrics and visual interpretation of trended data to guide intervention in favour of automated, alert-based, multiparametric algorithms to guide clinical interventions (see below). Second, it is potentially relevant that REM-HF enrolled patients with devices manufactured by different vendors. This assumes equivalence in the performance of HF diagnostics across all manufacturers, which in REM-HF varied significantly in parameters measured and preset thresholds. Finally, routine review of normal HF diagnostic data, as was performed and highlighted in REM-HF, is no longer encouraged as a high-burden, low-impact exercise.
Algorithmic, Multiparametric Heart Failure Remote Monitoring Tools
Remote device diagnostics for HF have advanced considerably over the past decade. Evolving technologies have led to iterative improvements and transition away from first-generation single metrics and second-generation multiple metrics to third-generation algorithmic multiparametric HF RM tools.
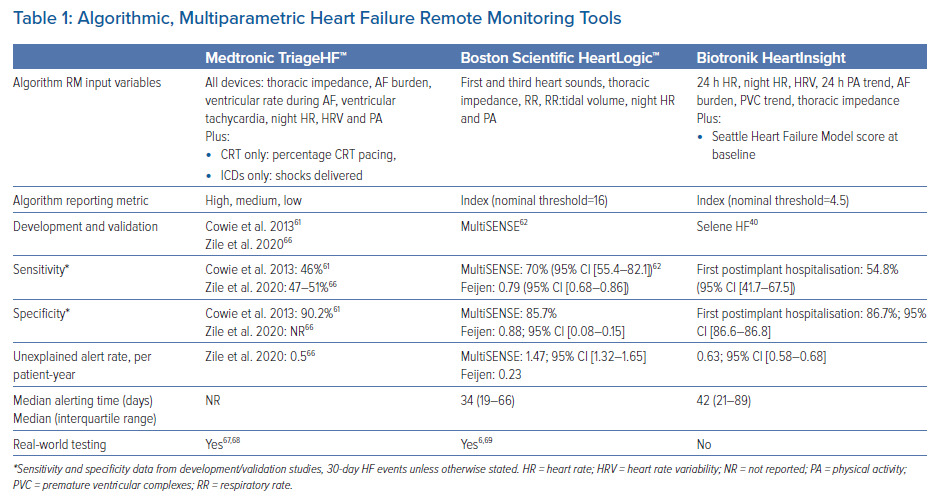
The two dominant algorithmic HF RM platforms currently available in the UK are TriageHF [Medtronic] and the HeartLogic index [Boston Scientific]. In addition, Biotronik released their HeartInsight index in April 2022. All three differ in parameters measured, procedures for data processing and alert generation (Table 1).
Medtronic TriageHF Risk Status
Previously known as the Heart Failure Risk Status, the TriageHF risk status is a multiparametric HF risk stratification tool based on a Bayesian model. Unlike other multiparametric algorithms, as TriageHF is calculated within Carelink (i.e in the cloud not the device), TriageHF alerts are available across all devices (CRTD, ICD and CRTP) with the Optivol 2.0 feature. This enables the technology to be backwards compatible with patients who have legacy devices already implanted.
Output is displayed as a traffic light system, with a low-, medium- or high-risk status indicating the risk of 30-day HF hospitalisation (Supplementary Material Figure 2). The algorithm was originally developed and validated by Cowie et al.61 following on from the PARTNERS-HF study, which highlighted the value of combined metrics over uniparametric monitoring.3 Cowie et al. combined data across multiple studies to show that patients with a high-risk status were 10-fold more likely to be admitted with HF in the subsequent 30 days than low-risk status patients (HR 10.0; 95% CI [6.4–15.7]; p<0.001). The combined score outperformed all single metrics (HR 10.0), including thoracic impedance (HR 2.6) and arrhythmias (HR 3.01).61 The risk status is calculated daily for each patient. CareAlert notifications (an automated real-time transmission to the provider) need to be programmed ‘on’ in order for TriageHF risk status data to be notified to clinical teams via CareLink, Medtronic’s RM platform. Using healthcare claims data and TriageHF data from over 20,000 patients, Zile et al. reported an unexplained detection rate of 0.5 per patient-year.66 Recent real-world data from a Manchester, UK, cohort found that 60% of HF hospitalisations were preceded by a high-risk status within 30 days.67
Beyond HF hospitalisation, recent studies have also examined the relationship between TriageHF status and all-cause mortality. One study examined data for 439 adults with TriageHF-compatible devices over a median 702-day follow-up. Spending ≥1 day in a high-risk status was associated with a 3.07 increase in the odds risk of death (95% CI [1.57–6.58]; p=0.002), with a correlation observed between the number of days in a high-risk status and the risk of death.68 Zile et al. reported that 4-year all-cause mortality rates for high- and low-risk status patients (maximum score within the first 6 months of score initialisation) was 38% and 14%, respectively.66
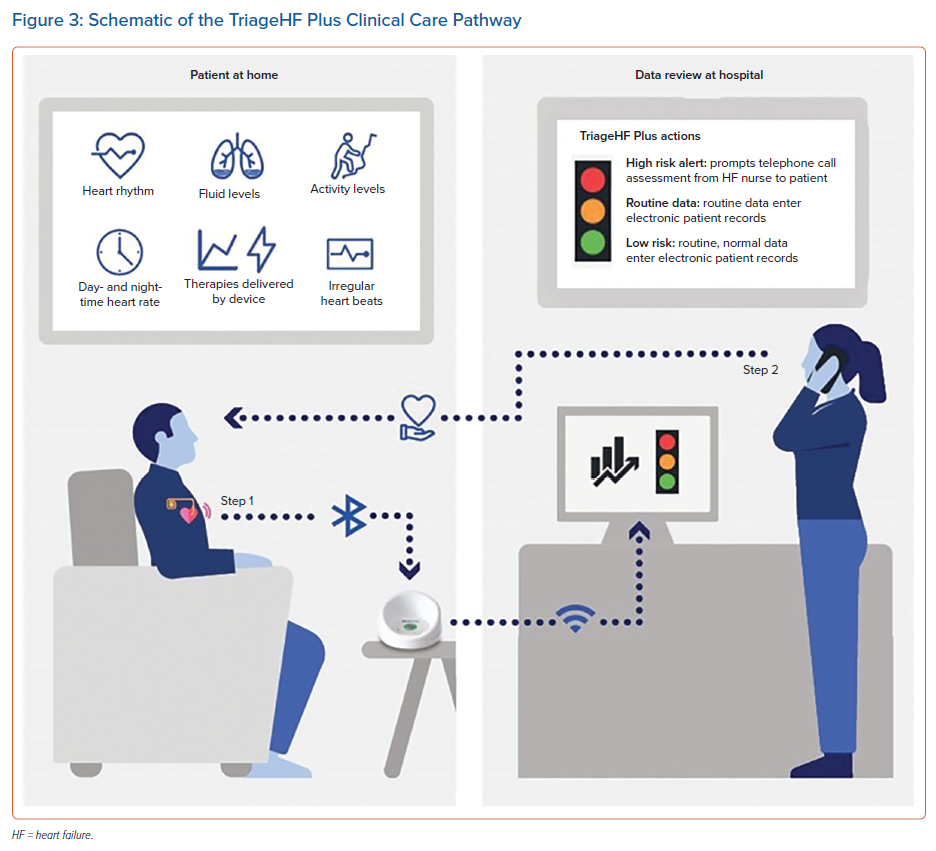
The TriageHF risk status has been tested in combination with a clinical pathway in various small-scale clinical studies. In each case, a high-risk status served as the primary trigger for telephone-based assessment and intervention (Figure 3). The sensitivity and specificity for detecting worsening HF (compared with clinical assessment) ranged from 83% to 99% and from 59% to 63%, respectively (Figure 3).47,70,71
Preliminary results from a real-world service evaluation study in Manchester comparing a cohort under a TriageHF based clinical care pathway with a comparator group reported a 58% lower all-cause hospitalisation rate across 14 months follow-up.72 Full results of this study are due later this year.
Boston Scientific HeartLogic Index
The HeartLogic index was developed and validated in the MultiSENSE study, which ran between 2010 and 2013.50 Of note, the index is restricted to the Resonate™ family of CRTD and ICD devices, as the algorithm sits within the device itself. Index parameters differ from TriageHF in that they do not include AF (unless there is a fast ventricular rate), but instead include respiratory features to suggest increased respiratory effort (respiratory rate and tidal volume). Additional features are available but are not part of the algorithm (sleep incline, AF/atrial tachycardia burden, shocks, pacing and weight via Bluetooth-connected external weighing scales). Metrics on individual parameters have not been reported. In all, 900 patients were recruited to the international MultiSENSE study, which included patients with compatible CRTDs into 12-month follow-up. Using a threshold of 16 (the current default setting to trigger an alert), the sensitivity of the HeartLogic algorithm to detect HF hospitalisations or visits requiring intravenous diuretic treatment was 70% (95% CI [55.4–82.1%]), with alerts being triggered, on average, 34 days prior to an HF event.50,69,73 A post hoc analysis of data from the MultiSENSE study reported a 10-fold increase in HF events when patients were in an alert state compared with when they were not in an alert state.73 Santani et al. reported real-world data in 2020, following a cohort of 104 patients with alerts activated for a median of 13 months.69 Patients were telephoned monthly, as well as additionally in response to an index above 16. In that study, 0.93 alerts per patient-year were received by the clinical team, with 60% judged to be clinically meaningful (43% requiring clinical action, including an additional 282 scheduled and 56 unscheduled clinical examinations).69 Of the alerts, 29% were unexplained.69 Feijen et al. reported on the integration of the HeartLogic with an HF care pathway using telephone-based clinical assessments (107 participants, 136 alerts over a median 14-month study period).6 Alerts were true positives in 71% of cases, with a sensitivity and specificity of 0.79 (95% CI [0.68–0.86]) and 0.88 (95% CI [0.08–0.15]), respectively.6 The MANAGE-HF randomised trial comparing HeartLogic ‘on’ versus ‘off’ is ongoing and due to complete in 2025 (NCT03237858).
Biotronik HeartInsight Index
Biotronik launched its multiparametric HF diagnostic algorithm in 2022. The development and validation of the HeartInsight algorithm was reported in the multicentre SELENE-HF study, which reported that the algorithm could predict two-thirds of post-implant HF hospitalisations, with a median time from alert to hospitalisation of 42 days.40 This algorithm combines real-time parameters (similar to TriageHF) with the baseline Seattle Heart Failure score, which the authors report did not affect algorithm sensitivity but, interestingly, did reduce false and unexplained alert rates by approximately 10%.40 The predictive value of other individual parameters was not reported. Patients with permanent AF were excluded from this evaluation.
Current and Future State of Play
Although an increasing number of centres are beginning to evaluate RM risk data from implanted devices, HF RM using device HF risk data is not in routine clinical use. UK guidelines do not currently recommend this form of monitoring given the lack of reproducible evidence of clinical impact in randomised studies. Most agree that HF remote monitoring should work; thus, the reasons for mixed results in randomised clinical studies continue to be hotly debated. There is increasing evidence from recent non-randomised and real-world studies indicating benefit.47,67,69 Table 2 summarises HF RM platforms available in the UK.
Two dominant methods of HF RM have emerged: algorithm-based multiparametric monitoring and PAP monitoring. These alternative approaches have not been tested head to head, and differences in data processing and clinical action procedures between systems limit direct comparison. PAP monitoring is arguably more specific to HF status, whereas multiparametric methods are more likely to detect a broader range of clinical scenarios requiring attention. Alerts based solely on thoracic impedance lack clinical accuracy.
Procedures for dealing with RM data are probably the easiest targets for improvements in efficacy. The ongoing prospective multicentre TriageHF Plus study is due to report late 2022, and will evaluate the workforce burden associated with the implementation of a pragmatic clinical pathway guided by RM TriageHF risk data.74 Real-world data from the MANAGE-HF study, a randomised control trial of HeartLogic, are also eagerly awaited.
Expanding access to RM device data beyond specialist device clinics may help integrate HF and device services. An example of this is the comanagement feature within Medtronic CareLink, whereby RM data are automatically shared with HF clinics. Other directions being explored are the integration of RM data into a multiparametric platform including laboratory results and app data, with one such example being the French Careline system.75 Patients are supplied with smartphones with Bluetooth-enabled blood pressure/heart rate monitors, weighing scales and patient symptom tracker application. This links with data from laboratory results and implanted devices to provide on-screen trend analysis to be viewed by the clinician (Supplementary Material Figure 2).
Other areas of interest are integrating RM with app-based platforms to communicate with patients alongside device data collection. This may take the form of questionnaires in response to deviated parameters to help with risk stratification or feedback information and advice to patients as part of a self-care programme.76
Device technology is also progressing rapidly, with new systems entering the market. One such example is the V-LAP monitoring system. This system is similar to the CardioMEMS system in design, but sits in the interatrial septum to directly measure LAP. The first in-human VECTOR-HF trial reported safety and accuracy in June 2022.77
Conclusion
There is increasing evidence that RM data from cardiac devices can be used to predict and prevent acute HF episodes. The ways in which data are managed and actioned vary by study, but there is a trend towards increased automation to reduce additional work burden on clinical teams. Real-world data are emerging, with numerous important studies due to report in the next few years. The optimal model of care to integrate RM data into established clinical pathways for HF patients is yet to be established, but triage-based telephone assessments triggered by RM ‘alerts’ appear to be a promising future standard of practice.
That said, device RM is not without issue, with concerns regarding additional clinical work, data overload and a lack of access to health data beyond specialist services. As clinical care pathways return to a form of normality, the extent to which elements of pandemic remote care will remain is unclear.
Patients are increasingly aware that their health data are monitored by cardiac devices and, as transmission technology improves, it feels inevitable that RM will become ingrained into care pathways. However, just because device health data can be collected does not mean it should be collected routinely without evidence of positive clinical impact. A scenario where health data are collected but not reviewed could be a dangerous one.
In 2019, before the COVID-19 pandemic, Brahmbhatt and Cowie argued that: “It is not difficult to collect data remotely, but it has been a challenge to find a way to integrate such potentially continuous data streams into systems of care, and to convert more data into better decision-making that improves the outcome or experience of care.”65
Patients, care providers and industry need to work together to build RM clinical pathways that are pragmatic, impactful, cost-effective and promote self-care.
Clinical Perspective
- Device heart failure remote monitoring capabilities are rapidly advancing, with various clinical tools available across manufacturers.
- Evidence to date shows remote monitoring data can identify patients at high risk of heart failure events in 30 days, with sensitivity and specificity in the range 46–70% and 86–90%, respectively.
- Real-world testing remains limited to small studies, but results so far are promising. All strategies have used structured telephone-based assessments, feeding into clinical pathways based on the temporary escalation of diuretic therapy and optimisation of guideline-directed medical therapy.
- Haemodynamic pressure monitoring (e.g. the Abbott CardioMEMS system) is the only remote monitoring system currently recommended in clinical guidelines applicable to the UK (symptomatic patients with heart failure with reduced ejection fraction).