Cardiovascular diseases and cancer pose a significant morbidity and mortality burden on a global scale.1–5 Advances in cancer therapeutics and treatment strategies have resulted in improved cancer outcomes, leading to a growing cancer survivor population. However, there has been a concerning uptrend of cardiovascular diseases, including ventricular arrhythmias (VA), in cancer patients.1,6 Determining the arrhythmogenicity of cancer therapies can be challenging because the standard protocols of testing in healthy individuals during pre-drug approval cannot be implemented for chemotherapeutic agents.7
Cancer-treatment-associated arrhythmia is a form of cardiotoxicity that is increasingly relevant and described in the literature, but poorly understood and characterised because of the heterogeneity of malignancies, multitude of cancer treatments, pre-existing cardiovascular risk factors and unclear arrhythmogenic mechanisms.2–5,8 The increasing age of the population with cancer, overlapping risk factors (hypertension, diabetes, hyperlipidaemia, inflammation and obesity) and history of cardiovascular diseases can predispose certain patients to a higher risk of developing arrhythmias during and after cancer treatment.3 In a recent evaluation of post-marketing safety communications for 125 cancer therapeutics approved by the Food and Drug Administration (FDA), arrhythmias were the most common reason for cardiovascular disease warnings (23.5%).8 Acute cardiotoxicities during treatment may preclude the administration of potentially life-saving cancer therapies, while chronic toxicities following treatment can impact long-term survival and quality of life. Arrhythmic cardiotoxicity can either be because of primary effects (i.e. the drug directly disrupting specific molecular pathways leading to development of arrhythmias) or secondary effects (i.e. damage to the cardiac structure through ischaemia, inflammation or radiation therapy, with VA as a resulting phenomenon). Additionally, the mechanisms underlying arrhythmias in cancer patients are also driven by a complex interplay of both cancer and non-cancer treatment adverse effects along with electrolyte disturbances (Figure 1).
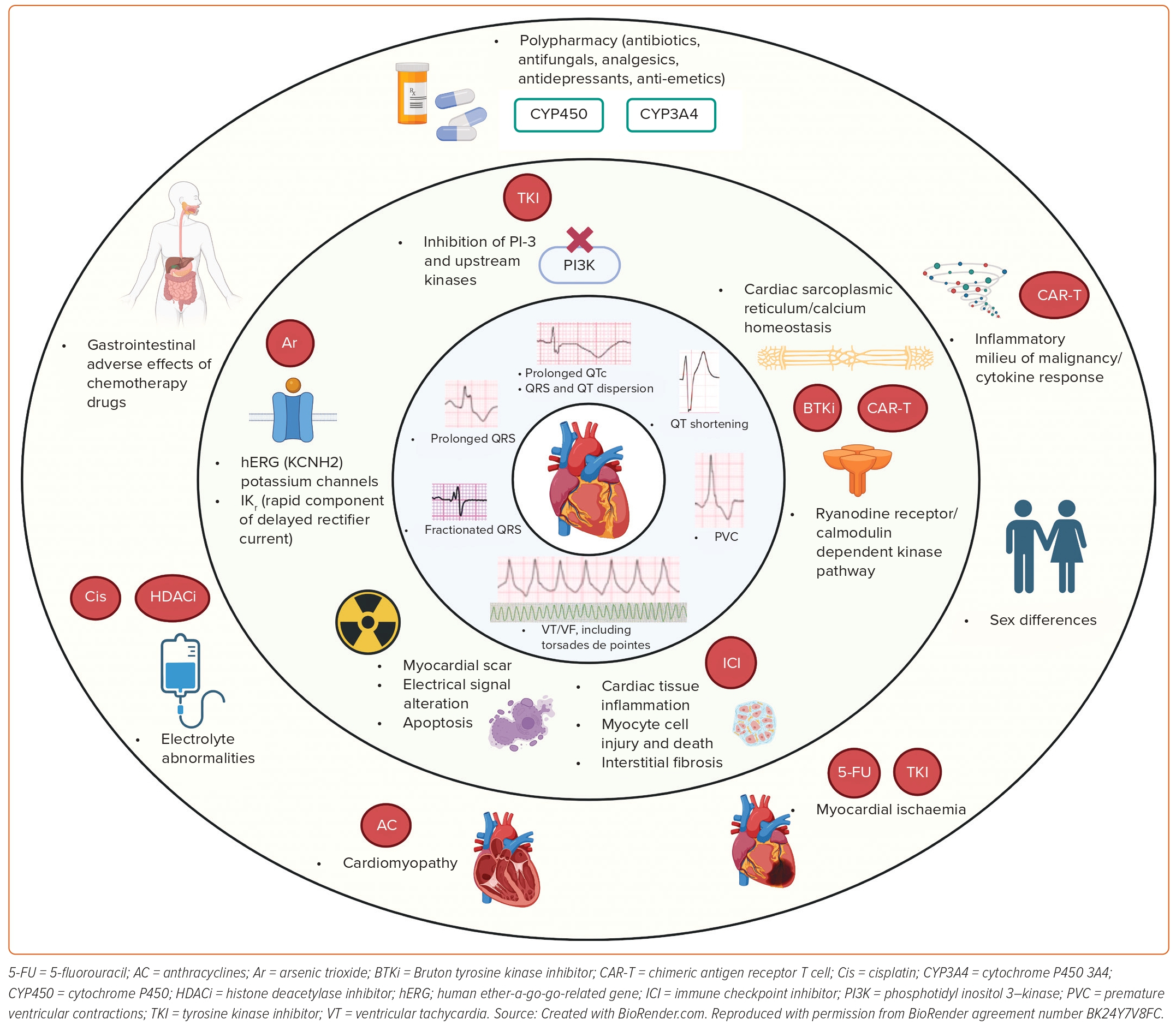
To illustrate the importance of understanding the causes of arrhythmogenicity related to the wide spectrum of cancer treatments, prior scientific statements and guidelines have been published.4,5 In this review, we will describe the known associations of traditional and novel cancer treatment agents with ventricular conduction (QRS) abnormalities (Figure 1). Next, we will review specific high-risk drug classes associated with QT prolongation and VA. Lastly, known approaches and strategies that can potentially help in the identification and prevention of cancer-treatment-induced VA will be discussed (Figure 2).
Ventricular Repolarisation Abnormality (QT Prolongation)
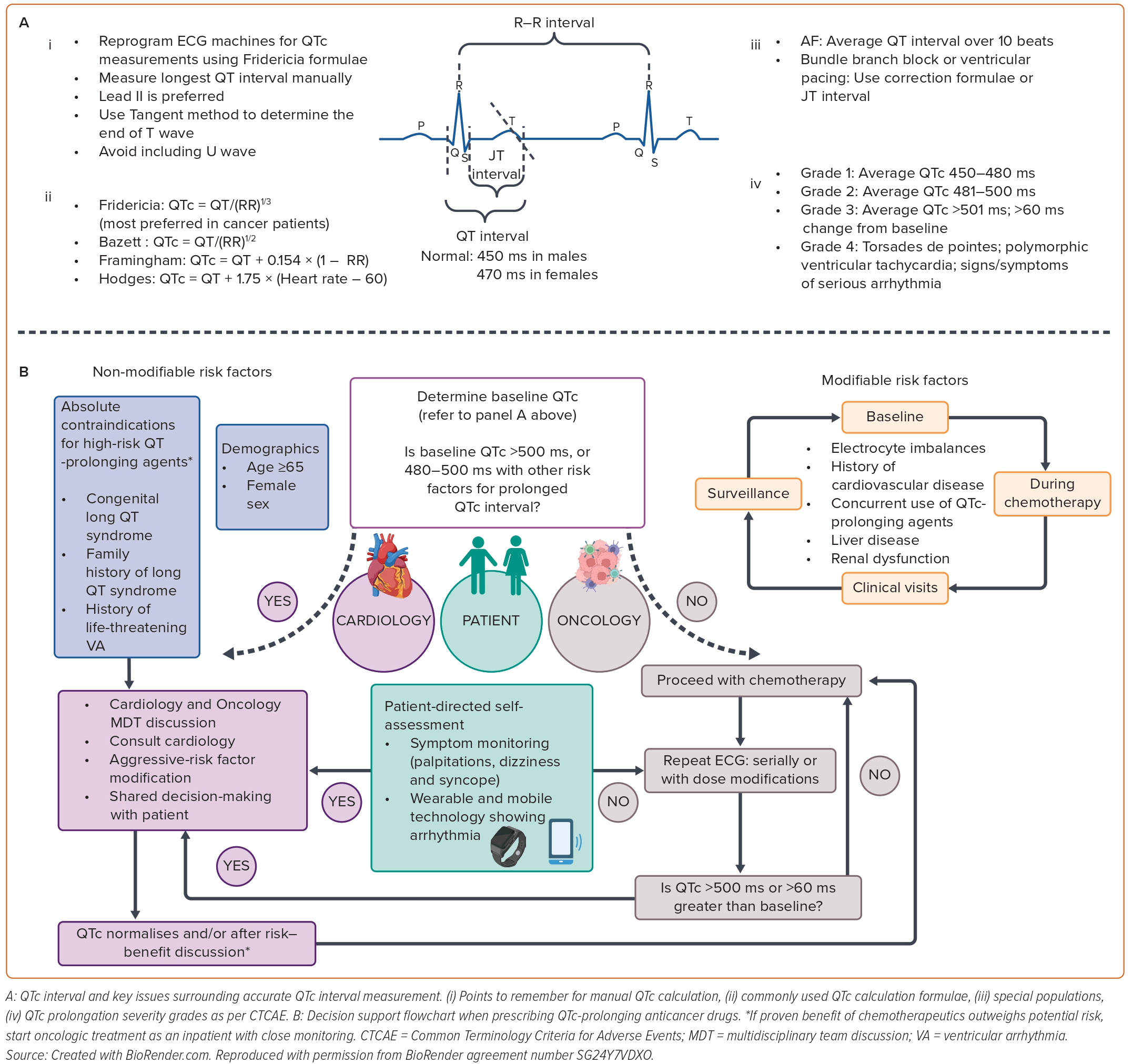
The QT interval, representing ventricular repolarisation, is the principal surrogate marker to assess the arrhythmic risk of a drug. The prolongation of cardiac repolarisation is reflected in ECGs by a prolonged QT interval that is corrected for heart rate using different methods (Figure 2).9 QT interval prolongation increases the risk of the specific VA torsades de pointes (TdP) leading to symptoms, sudden cardiac arrest and death. Because of this safety concern, all drugs undergo preclinical assessment in healthy volunteers for the risk of TdP as an off-target effect. However, in onco-therapeutics, these safety studies are conducted in target cancer patients with normal underlying corrected QTc intervals (QTc) and electrolyte values.10 Yet, a recent review of 205 anticancer drugs reported the presence of thorough QT/QTc assessment among only ~10% of these drugs (22 out of 205 drugs).11
QTc prolongation is very common in cancer patients receiving chemotherapy, with a reported incidence of up to 30%.12 Patients with cancer have been reported to be more susceptible to QTc prolongation compared with the non-cancer population. Potential explanations include direct sequelae of the cancer therapeutics causing changes in cardiac repolarisation from effects on the human ether-a-go-go-related gene potassium channel – now commonly referred to as KCNH2 – and the rapid component of the delayed rectifier potassium current, IKr.13 Other pathways, such as inhibition of phosphoinositide 3-kinases or upstream kinases, have also been reported to cause prolonged QTc.14,15 Additionally, confounding factors, such as electrolyte abnormalities, exacerbated by gastrointestinal adverse effects, such as nausea, vomiting and diarrhoea, as well as polypharmacy with medications, such as analgesics, antibiotics, antifungals, anti-depressants and anti-emetics, can potentiate QTc interval prolongation.
Given that potentially beneficial cancer therapy may be withheld because of perceived or overestimated risks of arrhythmia from QTc prolongation, it is important to meticulously assess the QT interval, before and during treatment, followed by accurate calculation of the QTc, recognising the weaknesses and strengths of the various formulae (Figure 1).16 Borad et al. studied the impact of different QTc formulae (including Bazett’s and Fridericia) in cancer patients on clinical trial enrolment eligibility and demonstrated disparate results leading to ineligibility rates ranging from 3.1–17.7% (3.9% based on Fridericia’s formula versus 10.8% using Bazett’s formula).17 Similar observations of QTc interval overestimation from Bazett’s formula and better predictive ability of Fridericia’s formula has also been reported by others.18 Therefore the Fridericia correction method for QTc calculation is recommended in cancer patients because of higher accuracy.11 Additionally, careful interpretation of the QTc interval is warranted when the heart rate is irregular or in the case of a pre-existing bundle branch block or pacemaker rhythm (Figure 2).
Ventricular Conduction Abnormalities: QRS Fragmentation and Prolongation
The QRS duration corresponds to ventricular depolarisation, and monitoring of QRS duration is routinely used in care of patients who are at risk for developing arrhythmias and heart failure.19 Electrical depolarisation abnormalities, defined as QRS duration of ≥120 ms, can be a sequelae of chronic or acute conduction system disease, myocyte-cell injury, cardiac tissue inflammation and interstitial fibrosis.20 QRS prolongation, both prior to initiation of chemotherapy and during treatment, has been shown to be associated with increased risk for cardiac dysfunction, VAs and major cardiovascular events.21 Hence, a prolonged QRS can be used to recognise sub-clinical cardiac damage and potentially trigger cardiac-risk stratification of patients on chemotherapy, impacting the acute and long-term outcomes.21
QRS fragmentation (fQRS) is a marker of ventricular depolarisation abnormality and can be due to alteration of electrical signals traversing the myocardium distorting QRS morphology. This has been reported in patients with myocardial scar after MI and is a potential non-invasive marker for identifying patients at risk for sudden cardiac death (SCD).22,23 The prevalence of fQRS ranges from 1% to 30% in the general population, with a much higher prevalence in cancer patients after treatment with chemotherapy.24 Given that myocardial fibrosis is an adverse effect of cancer therapy and chemotherapies can trigger apoptosis or necrotic myocyte death, studies have reported the importance of fQRS to recognise chemotherapy-related cardiotoxicities even preceding development of cardiac symptoms or echocardiographic abnormalities.25,26
Ventricular Arrhythmias
Metabolic changes at a cellular level in patients with cancer, due to both the catabolic state of cancer and the treatments they receive, can predispose to VA. As per the National Cancer Institute’s Common Terminology for Cancer Adverse Events (CTCAE – Version 5.0), there are four grades of severity of cardiac events: Grade 1: asymptomatic, intervention not indicated; Grade 2: non-urgent medical intervention indicated; Grade 3: urgent medical intervention indicated, Grade 4: life-threatening consequences, haemodynamic compromise. The QTc interval is used as a proxy for drug-induced VA risk, although it is imperfect.14 Certain treatments, such as Bruton tyrosine kinase inhibitors (BTKi) and chimeric antigen receptor T-cell (CAR-T) therapy, have been associated with VA without concomitant QT prolongation. The mechanism has been attributed to alterations in cardiac sarcoplasmic reticulum Ca2+ homeostasis associated with cardiac ryanodine receptor-calmodulin-dependent protein kinase pathways.27,28 While potential complications of VA and sudden cardiac arrest are serious in nature, actual arrhythmic events associated with QTc prolongation are rare.29
VAs, such as premature ventricular contractions (PVCs) and non-sustained ventricular tachycardia (NSVT), have been reported to have higher prevalence in patients with cancer than in those without cancer (8.0% versus 0%).30 This may plausibly be attributed to factors, such as heightened clinical surveillance, the inflammatory milieu of malignancy, as well as the use of cardiotoxic chemotherapeutic agents. Among 120 patients with lung, colon and pancreatic cancers, NSVT [HR 2.4] and PVCs (HR 1.0) were both significant independent predictors of mortality during long-term follow-up.30
Several published case reports and case series have reported the occurrence of VA (both low grade, such as PVCs or brief NSVT episodes and malignant ventricular tachycardia [VT]/VF) and SCD in patients with cardiac tumours.31,32 Overall, cardiac tumours are a rare cause of arrhythmias, although the true prevalence of primary cardiac tumours causing SCD is unknown and may likely be underestimated as post-mortem analysis is not performed on all patients.33 PVC morphology reflects the chamber of origin; right bundle branch block pattern is seen in 80% cases (left-ventricular origin) and left bundle branch block is present in 20% cases (right-ventricular origin).33 Probable mechanisms include tissue compression, mass effect (involving cardiac cavity, coronary vasculature or obstruction of heart valves and outflow tracts), extensive bleeding into the lesion leading to tamponade, scarring and creation of re-entrant pathways producing VT and asynchronous refractoriness within the abnormal tissue leading to electrical instability.33,34
Cancer Treatments Associated with QTc Prolongation and Ventricular Arrhythmia
Arsenic Trioxide
Arsenic trioxide, used for treatment of otherwise fatal acute promyelocytic leukaemia (APL), has been reported to cause prolongation of the QT interval (30–90% incidence) and TdP at therapeutic doses.35 Mild prolongation of the QT interval (440–500 ms) is commonly seen and should not limit the use of arsenic trioxide. It is recommended that ECGs be obtained at baseline and during therapy (weekly monitoring) along with close electrolyte monitoring. A QT interval >500 ms, should trigger aggressive correction of abnormal serum potassium, magnesium, calcium and creatinine levels, and consideration of alternative treatment regimens if significant QT prolongation persists. In the presence of clinical symptoms (syncope, palpitations or irregular heartbeat) even with a QT interval <500 ms, hospitalisation for cardiac monitoring should be considered with a plan to resume arsenic trioxide therapy only after resolution of symptoms, QTc <460 ms and electrolyte correction. Alpha lipoic acid and mexiletine may help to minimise arsenic-induced QT prolongation and recurrent TdP, although further research is needed to determine the efficacy and safety of this approach.36–38
Anthracyclines
Anthracyclines (AC) are some of the most effective and widely used chemotherapeutic agents. Anthracycline-induced QT prolongation and TdP has been reported in patients even within a safe dose range and weeks to years following treatment.39,40 Interestingly, the incidence of QTc prolongation increases with subsequent AC cycles and can lead to VA.41–43 Both electrocardiographic changes (ST-T segment changes, QRS voltage lowering, T-wave flattening, QTc interval prolongation) and VA following AC administration have been reported in ~30–66% of patients with occurrence even during the first cycle of therapy.41,44 fQRS has been reported in AC treated patients with breast cancer (27%) and large B-cell lymphoma (29%).45,46 The prevalence of VA in AC-related cardiomyopathy has been shown to be similar to non-cancer-related cardiomyopathy.47 Dexrazoxane, an FDA-approved drug for patients with metastatic breast cancer (with cumulative AC dosages above a threshold of 300 mg/m2 for doxorubicin dose, or >550 mg/m2 for epirubicin) may be used to protect the heart from anthracycline-induced QT prolongation.48
Antimetabolites
5-fluorouracil (5-FU), is commonly used in gastrointestinal and breast cancers, and has been shown to cause wide spectrum of VA ranging from frequent PVCs to SCD.49 A study reported incidence of VT in 3.7–7.4% of patients, primarily driven by a 5-FU-related ischaemia mechanism.50
Histone Deacetylase Inhibitors
Panobinostat, a treatment for refractory multiple myeloma, carries a black box warning related to severe arrhythmias and ECG changes, such as QTc interval prolongation. QTc prolongation >60 ms or an absolute value >500 ms was noted in 8.6–28.0% of castration-resistant prostate cancer patients.51 Romidepsin, used for cutaneous T-cell lymphoma, is also associated with QTc interval prolongation, VT and SCD. A phase II study of romidepsin in patients with metastatic neuroendocrine tumours was terminated prematurely because of concerns about SCD.52
Cisplatin
Cisplatin is a platinum-based compound used in metastatic gonadal tumours and advanced bladder cancer. Cisplatin-related hypomagnesaemia and QT dispersion due to sodium channel effects have been associated with VA, such as NSVT, with an incidence as high as 8.0%.41
Ivosidenib
Ivosidenib, an isocitrate dehydrogenase enzyme inhibitor, is used for the treatment of acute myeloid leukaemia (AML). In a Phase I study of 258 ivosidenib-treated AML patients, QT prolongation was noted in 7.8% of the overall study cohort. This led to dose interruptions in 13 patients; 12 patients had a serious non-fatal adverse event of QT prolongation.53
Tyrosine Kinase Inhibitors
Tyrosine kinase inhibitors (TKIs) have been implicated in VA by enhancing automaticity through increased early and late after depolarisations.54 Nilotinib is a BCR-ABL tyrosine kinase inhibitor used in the treatment of chronic myeloid leukaemia. It carries a black box warning for QTc prolongation and SCD. Studies have reported that up to 26.0% of patients treated with nilotinib develop QT prolongation longer than 30 ms.55 Dasatinib use has been associated with high burden of PVCs and new arrhythmias occurred in ~11% of patients while QTc prolongation >500 ms was seen in 2.0% of patients.56,57
Vandetanib is a kinase inhibitor of vascular endothelial growth factor receptor, epidermal growth factor receptor and RET-tyrosine kinase. It is used to treat surgery-ineligible medullary thyroid cancer patients and breast and lung cancer patients. The overall incidence of QTc prolongation has been reported as ranging from 16.4% to 18.0%.58 In a meta-analysis of 18 clinical trials, vandetanib was associated with a statistically significant risk of QTc prolongation, with increased risk at higher dosages (RR 10.6 versus 4.8 for lower doses).52
Ibrutinib is a BTKi used in the treatment of chronic lymphoid leukaemia, mantle cell lymphoma and Waldenström’s macroglobulinaemia. Ibrutinib’s role in VAs and SCD has been rapidly evolving.59,60 In randomised controlled trials, the incidence of all-grade VAs was 1.0% versus 0.4% in among controls. In analyses from a US-based comprehensive cancer registry cohort, ibrutinib-treated patients without baseline heart failure or coronary artery disease had higher rates of VA than non-ibrutinib-treated subjects (596.0 versus 48.1/100,000 person-years) with an observed versus expected RR of 12.4 (p<0.001).59 Interestingly, the risk of VA appears to be dose-related based on one study that used the international pharmacovigilance database VigiBase and performed a disproportionality analysis.28 Among the approximately 81.4% of the patients on ibrutinib who experienced VAs, 91.0% were taking at least 420 mg of ibrutinib per day, and only 9.0% were taking 280 mg or lower per day. Lampson et al. analysed the FDA Adverse Event Reporting System (FAERS) for reports of VAs in patients receiving ibrutinib and found at least seven instances of VT/VF and 6 sudden deaths.60 Also, among 1,000 clinical trial participants in an ibrutinib study, 10 cases of sudden death or cardiac arrest were identified.60 VAs have been suggested as a class effect of BTKi therapies.61 In an exploratory retrospective analysis of 290 B-cell lymphoma patients treated with acalabrutinib, nearly 3.0% developed cardiotoxic VA events, with one sudden death, independent of structural heart disease and prior ibrutinib exposure.61
Cyclin-dependent Kinase 4/6 Inhibitors
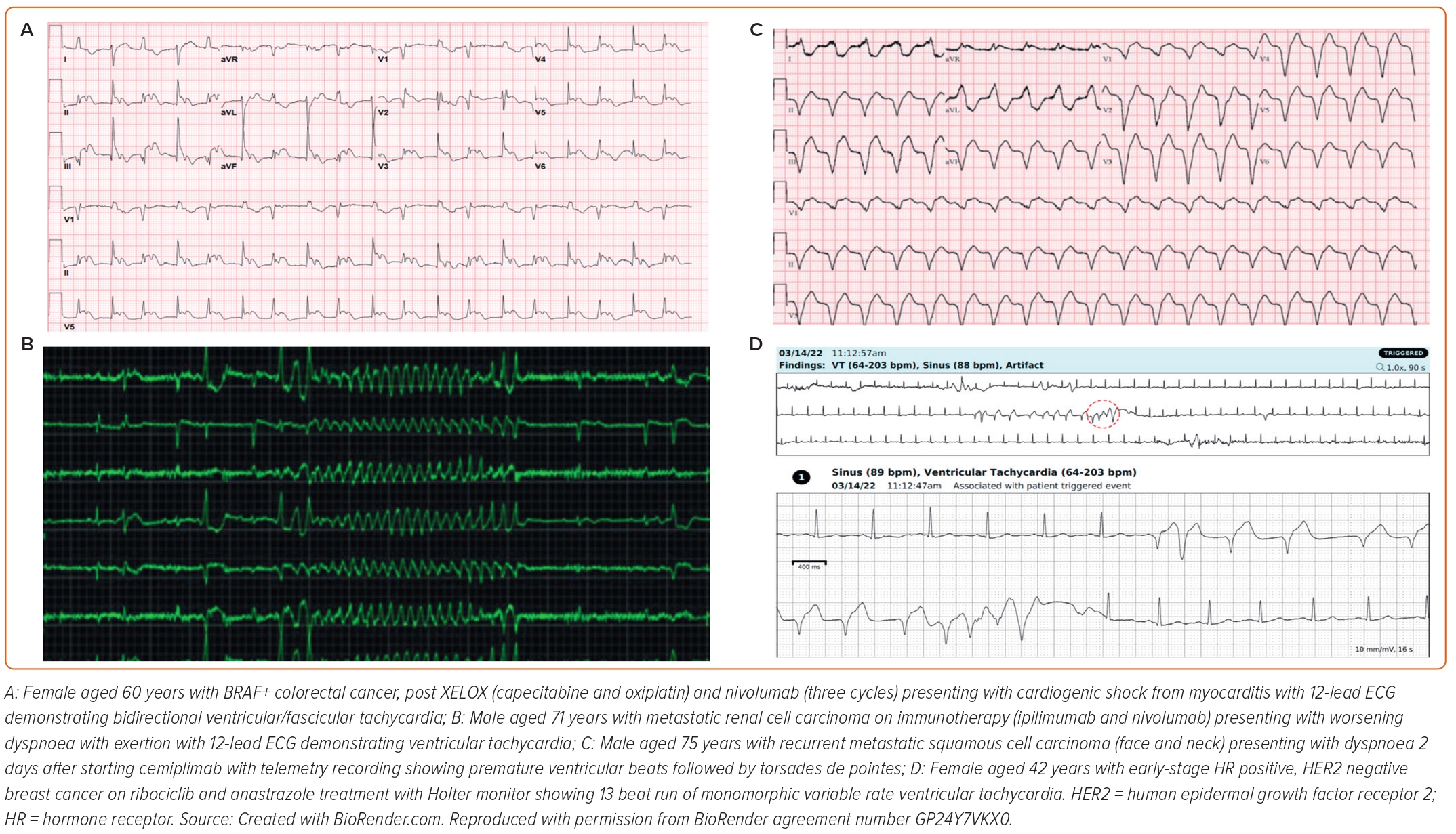
Cyclin-dependent kinase 4/6 inhibitors are used to treat hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancers. In its landmark Phase III clinical trial, ribociclib was associated with QTc prolongation in 11/334 (3.3%) versus 1/334 (0.3%) in the placebo arm.62,63 Figure 3 shows a Holter recording of VA in a ribociclib-treated breast cancer patient.
Immune Check Point Inhibitors
Immune checkpoint inhibitors (ICI) have revolutionised the field of chemotherapeutics, targeting a range of costimulatory signalling molecules on T lymphocytes and antigen-presenting cells, such as cytotoxic T-lymphocyte antigen-4 and programmed cell death 1/ligand 1. They can cause myocarditis, which is a highly arrhythmogenic state sharing similar histopathological findings of inflammatory infiltrates and myocardial necrosis (CD8+ T cells interspersed with CD4+ T cells and macrophages) as seen in higher grade allograft heart transplant rejection. The risk is even higher with ICI combination therapies.64–67 ICI-related myocarditis has a fatality rate of 30–50%, with notably higher mortality in patients with VA.68 A small size observational study of 30 patients reported VA in 27% of patients with ICI-associated myocarditis with VA listed as cause of death in more than one-third of patients (3/8 cardiovascular deaths).66 QRS duration may also be prolonged in the setting of ICI-myocarditis and a QRS>110 ms is associated with major adverse cardiovascular events.21 A recent meta-analysis of 51 clinical trials reported a rate of 3.1% cardiovascular adverse events for ICI monotherapies and 5.8% for combination therapies.69 A multi institutional retrospective registry of 147 cases of ICI-myocarditis reported the presence of life threatening VA among 22/147 (15.0%) patients.65 Figure 3 shows ICI-related VA ECG and telemetry findings of three different patients.
Chimeric Antigen Receptor T-cell Therapy
CAR-Ts are genetically engineered T-cells programmed to identify tumour antigens and induce a cytotoxic immune response. Goldman et al. found tachyarrhythmias to be the leading cause of cardiovascular and pulmonary adverse events among 2,657 patients from FAERS system.70 VA constituted 18.9% of tachyarrhythmia cases and interestingly the presence of tachyarrhythmias correlated with development of cytokine release syndrome.70 Recently, Thotamgari et al. reported VT to be present during 4.7% of hospitalisations of patients undergoing CAR-T therapy and cardiac arrhythmia to be associated with significantly increased in-hospital mortality in these patients.71
Prevention and Treatment Strategies
It is of utmost importance to identify and modify factors that can contribute to VA in patients with cancer (Figure 3). The primary prevention strategy includes clinical history taking and review of a baseline ECG prior to chemotherapy initiation. For example, assessment of proarrhythmic factors including a history of prior TdP, cardiovascular disease, older age (>65 years), female sex, a family history of (or genetic predisposition to) congenital long QT syndrome, a prolonged baseline QTc, hypothyroidism, and reduced kidney or liver function and the use of other QT prolonging drugs should be performed.11 Polypharmacy in cancer patients is common because of the need for anti-nausea, antibacterial and antifungal agents for treatment and/or prophylaxis. Prescribing physicians should be educated about resources, such as CredibleMeds database (https://crediblemeds.org), that may be used as a source for the most recent information regarding risk for QTc prolongation or TdP.72 Efforts should be made to increase awareness and validate VA findings in adverse-event capturing databases, such as FAERS, VigiBase and the process to file FDA Form 3500.73–75 For novel therapeutics with unknown QT-prolonging effects, ECG monitoring after the first dose, at steady state and after each change in dose should be pursued.
If significant QTc prolongation is noted (QTc >500 ms or a change from baseline >60 ms), modifiable risk factors, such as electrolyte abnormalities, should be promptly corrected and non-essential medications should be carefully reassessed for risk and benefit profile.76 Also, patient education and counselling regarding identification of symptoms, such as palpitations, presyncope and syncope, with a clinical care plan to seek emergent evaluation should be formulated. In high-risk patients, interventions, such as use of mexiletine (as in the setting of arsenic therapy), may help to minimise drug-induced QT prolongation, and recurrent TdP.38 Malignant VA (VT or TdP) should be promptly treated with standard approaches including intravenous magnesium sulphate, maintenance of chronotropy with either isoproterenol or temporary transvenous pacing, lidocaine and advanced cardiac life support procedures as needed and withdrawal of the causative agent, if necessary.76,77 In cases of tumour invasion causing VA, surgical excision can be considered.78
While the prevalence of cancer patients undergoing cardiac implantable electronic device implantation (CIED) has significantly increased, the presence of active cancer has been associated with increased mortality and complications in CIED patients due to mechanisms related to immunosuppression, cardiotoxic chemotherapies, direct metastases to the myocardium and conduction system, fluid and electrolyte abnormalities and vagal reflex from emesis and radiotherapy.79 Competing mortality risks should be considered before defibrillator (ICD) implantation and generally, an ICD is deferred if there is not an expectation of meaningful survival for at least 1 year.80 In patients with VA predisposing risk factors, such as cardiomyopathy, evaluation for CRT in those with QRS >120 ms can help to reduce the desynchrony and improve outcomes.81
Future Directions
The cardiac electrophysiological properties of cancer drugs need to be more rigorously assessed during drug discovery pathways to help prevent significant cardiac morbidity and mortality in the real-world setting. Currently, huge inconsistencies exist in the collection and reporting of cardiovascular data in such trials. A universal consensus nomenclature, definitions and protocols agreed upon by both oncologists and cardiologists are urgently needed. This framework will help to establish and thereafter uniformly analyse real-world dynamic arrhythmia registries. Such data repositories will help to focus on prediction, risk stratification, preventive measures and eventually may lead to the discovery of vital cellular signalling pathways and safer oncologic drugs. The field of cardiac arrhythmia is on the cutting edge of advanced digital technologies, medical device development and wearables; healthcare application will help provide new information. Leveraging such innovative data with machine learning and artificial intelligence to collect cardiovascular metrics or to detect arrhythmias may be beneficial for all patients and specifically patients who have cancer.
Conclusion
In this review we have summarised the literature regarding ventricular conduction and repolarisation abnormalities in patients with cancer. VA – especially PVCs and other abnormalities, such as QTc prolongation – are a commonly encountered problem but can be managed in the majority of cases without significant treatment breaks by modification of surrounding factors, such as electrolyte corrections and meticulous revisions of concomitantly administered medications. Cancer patients have several factors that may cause or exacerbate a variety of VA and some cancer therapies may cause a significant increase in the risk of developing VA. Awareness of chemotherapies, surrounding modifiable factors, high-risk features and mechanisms that may predispose to such arrhythmias is important. The attention to risk factors and underlying comorbidities may facilitate prevention and risk reduction. Still, much remains unknown about the overall incidence, mechanisms and optimal methods to reduce risk in the field of cancer-treatment-related VA. There is an urgent need for more basic, translational, and digital innovative technologies-based research cross-sectioning clinical and scientific collaborations between oncologists and cardiologists to develop evidence-based strategies for management of this special and vulnerable population. 
Clinical Perspective
- The mechanisms of cancer-therapeutic-induced ventricular arrhythmia are multiple and are poorly understood.
- It is important for cardiologists to be aware of the association of individual chemotherapeutic agents with ventricular conduction/repolarisation abnormalities and arrhythmias to identify drug-mediated arrhythmias early and treat them effectively.