Ventricular Arrhythmias and Sudden Death: What Are the Knowledge Gaps and How Can Artificial Intelligence Address These?
Sudden cardiac arrest (SCA) is a major public health problem worldwide. In the US alone, ~360,000 individuals have SCA every year.1,2 SCA is a mostly lethal event, and despite the developments in acute management, at least 90% of the cases still lead to death (i.e. sudden cardiac death [SCD]).3 Given the low survival rate after an SCA event, accurate long-term prediction and prevention of these events are the key components to reducing the burden of SCA.
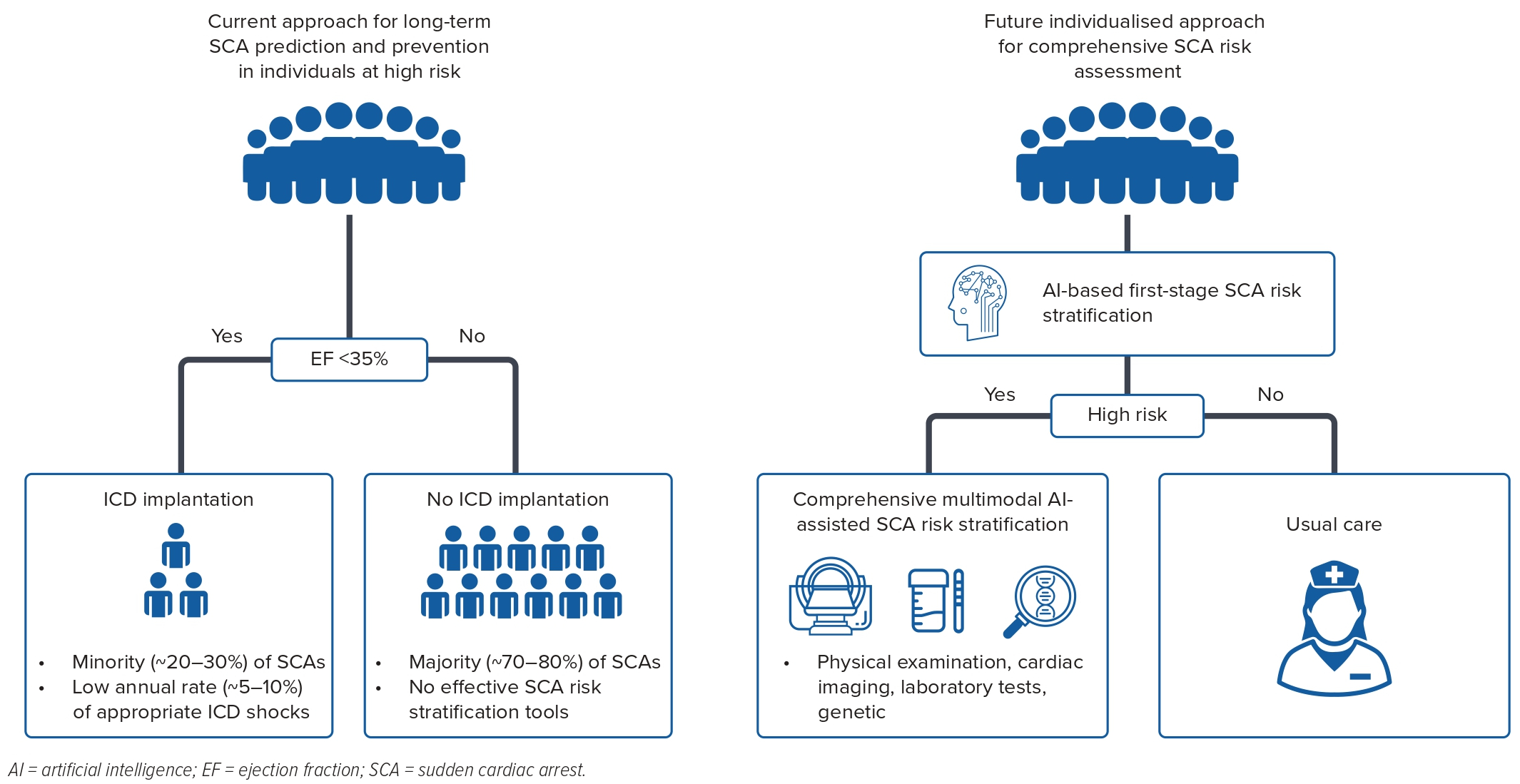
Current guidelines recommend long-term prevention of SCD for those with a severely reduced left ventricular ejection fraction (LVEF; <35%, primary prevention) or a history of VF or ventricular tachycardia (secondary prevention).4 The key limitation of this approach is low sensitivity, given that ~70% of SCAs occur in patients who are outside these recommendations.5 Moreover, at the present time <5% of ICD recipients receive appropriate ICD shocks per year, and the majority do not benefit from the device.6–10 Forty years ago, shockable rhythms accounted for the majority of SCA events in the general population as well as in hospitalised patients.11 However, there has been a major reversal in recent decades, and non-shockable rhythms now dominate, comprising 70–80% of the cases.12,13 As a consequence, it has become important to deploy artificial intelligence (AI) tools that will distinguish the shockable subgroup of SCA. There is a critical need to identify novel predictors of SCA in individuals with LVEF >35%. Furthermore, given the diminishing returns of the primary prevention ICD in those with LVEF <35%, it is important to augment and refine risk stratification for this subgroup as well.
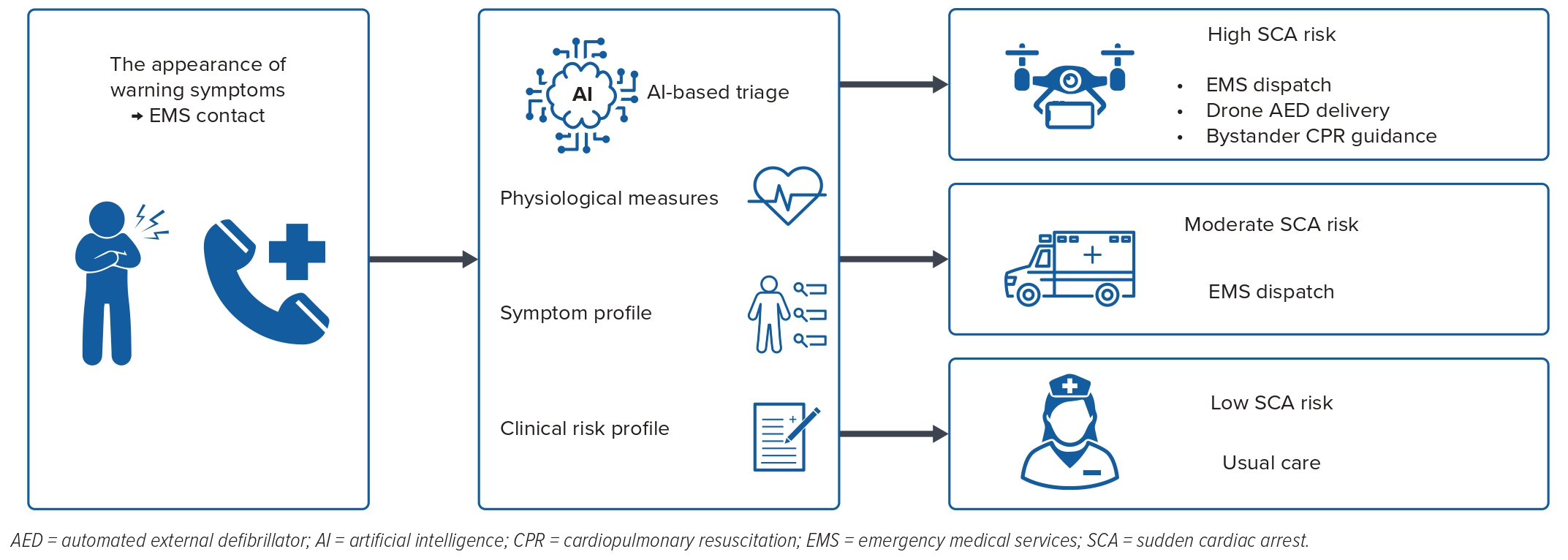
In addition to ICD-based long-term prevention of SCD, near-term prevention of SCA has been gaining scientific traction.14 Although SCD is generally considered a sudden and unexpected event, recent studies have demonstrated that more than half of the patients experience warning symptoms hours–days before the event.15–17 Although only a minority of these patients call emergency medical services, early contact may increase the likelihood of survival as much as fivefold.15 Near-term SCD prediction has the potential to prolong the window for prearrest management as well as shorten the delay for appropriate cardiopulmonary resuscitation and defibrillation. AI algorithms have a potentially important role in identifying symptom clusters that could improve prediction of near-term prevention with the promise of improving real-time triage using digital health technology.18
Methodology of Artificial Intelligence
AI is a broad term, and in general it refers to the simulation of human intelligence in computer systems that are programmed to mimic human actions.19 Machine learning (ML) is a subcategory of AI, and ML can be further categorised as supervised or unsupervised.20 Supervised ML is an iterative process that uses various data transformation algorithms to define the relationship between input data and labelled output data (e.g. predicting SCA). Before performing tasks in an unseen dataset, all ML models require a learning phase, in which inputs and outputs are used to train the model. After the model parameters are set in this learning phase, the ML algorithm has been developed and is ready to be applied to a previously unseen dataset (i.e. test set). Some of the most used supervised ML algorithms include linear regression, logistic regression, support vector machines, decision trees, random forests, neural networks and extreme gradient boosting. In contrast to supervised ML, unsupervised ML does not require labelled output data and the algorithm attempts to find patterns and create clusters in unlabelled datasets. The potential advantage of the unsupervised approach is that the clustering is not biased or forced to predict a specific output, which may enable better generalisation. Common unsupervised ML algorithms include k-means clustering, k-nearest neighbours, principal component analysis, factor analysis and hierarchical clustering.
Deep learning (DL) is a subcategory of ML that uses neural networks in more than three layers (deep neural network).21 The training of supervised DL algorithms is conducted similarly to supervised ML models. However, in contrast to most ML models, DL models may not require as much manual feature engineering (extraction and selection of specific features from the data before the learning process) as ML models. The strength of DL models is the ability to identify novel associations between input datasets and output, but DL models usually require larger datasets and higher computational power than ML algorithms.
SCA is a complex trait that can involve multiple underlying substrates and maintainers of ventricular arrhythmia (e.g. inherited conditions, coronary artery disease [CAD], diabetes etc.) and acute triggers (e.g. ischaemia, electrolyte imbalance). Such diverse and complex pathophysiology in which there may not be linear relationships between multiple data domains can create significant challenges for conventional SCA risk stratification methodology. In contrast, ML algorithms may provide the ability to identify novel patterns and clusters of SCA-defining variables in large datasets. At the present time, AI tools are deployed in single domains, for example ECGs or echocardiogram datasets. In the future, the development of multimodal AI tools that could potentially combine data, images and signals remains an exciting prospect to advance SCA risk stratification.22
Special Considerations When Using Artificial Intelligence for SCA Prediction
Sample Size
A common challenge in the development and training of an AI model is the availability of a sufficiently large and diverse dataset for training, which is crucial to achieving good model accuracy and generalisability. Overfitting is an important problem in which a complex model learns the training dataset too well and achieves perfect performance in the training set but performs poorly in the unseen testing dataset (poor generalisability). Common reasons for overfitting are small training sample size and complex algorithms that can learn all of the details/noise and fit the training data exactly but do not generalise to new data. Possible ways to overcome overfitting include increasing training data size and diversity, as well as reducing the model complexity. The goal is to develop and train a model that has good generalisability.
AI models are usually internally evaluated and validated in held-out test sets, which are part of the same original dataset from which the training set was drawn (e.g. data from one hospital). However, institutional and demographic factors may affect the generalisability. Hence, internal validation does not guarantee good external validation, and models should ideally be validated in an independent dataset that is collected separately (e.g. data from a different hospital).
Assembling a dataset with a sufficient number of SCA events suitable for the training of AI systems is challenging. Although SCA is a devastating condition, it affects approximately 50 per 100,000 in the general population. As a result, event rates are too small in cohort studies and the use of studies of existing cohorts with a low prevalence of SCA may lead to imbalanced datasets.2 Although the goal is to predict the minority class (SCA), data imbalance could introduce a bias towards non-events, potentially reducing the sensitivity for SCA detection. The problem of low event incidence and imbalanced data can be solved with a case–control approach. When collecting a valid control group, it is important to recognise that SCA patients are not healthy patients but instead have an underlying cardiac disease that ultimately led to SCA. Hence, it would be ideal to compare SCA cases to controls with underlying heart disease, especially CAD, instead of comparisons with healthy controls. If an AI model is developed for screening patients at high risk for SCA, the profile of control subjects is important in determining the target population.
Model Performance
Model performance metrics may also require special interpretation in the SCA context. Although an AI model may have a good discriminative value for SCD, a relatively low SCA event rate in the general population usually leads to a low positive predictive value. Moreover, good discrimination does not necessarily translate into good calibration, which is important for avoiding false interpretations and poor clinical decisions. For example, if a model correctly predicted SCA with a probability of 90% and another model predicted the same SCA event with a probability of 70%, both may be considered to be correct (with a 50% threshold), but the first model has better calibration. High SCA risk often correlates with a high non-sudden death risk as well, and identifying patients at a high risk of SCA without a similarly elevated risk of competing modes of death is important for accurate prediction and prevention.23
Phenotyping
Another key consideration in AI-based SCA prediction models relates to the definition of SCA. It is important to recognise that SCA is not a single disease and that affected individuals tend to have a combination of conditions such as coronary disease, heart failure, hypertension and diabetes. Although cardiac aetiologies are responsible for most sudden deaths (i.e. SCD), a proportion of sudden deaths are due to non-cardiac causes (e.g. aortic dissection, stroke, pulmonary embolism).24,25 This is an especially important aspect of in-hospital cardiac arrests.2 Moreover, given the differences in the treatment of shockable and non-shockable SCA, more specific SCA prediction based on presenting rhythm would be clinically useful in guiding preventive ICD implantation and preparing an appropriate first response to impending SCA.
Input Data
AI model performance is strongly dependent on data quality. In the medical field, input data can include tabular data from electronic health records (EHRs), images, or physiological signals. Each data type has specific strengths and weaknesses. Tabular EHR data may be noisy (having many errors and irrelevant data), have missing data, and require substantial preprocessing and feature engineering before it can be provided to an ML model. However, tabular EHR data-based ML models have good explainability, which refers to uncovering the underlying rules or importance of specific features for individual patients.
Deep neural networks can analyse more complex high-dimensional data such as images and physiological signals.26–29 There is less need for feature engineering, and deep neural networks can detect novel data indices that may lead to better model performance. However, data preprocessing is often needed, which refers to preparing raw data in a format that can be supplied to the model (e.g. resizing images). Deep neural networks are often called ‘black boxes’ because the data features that DL models select to predict the output may not be fully comprehensible to users. This reduces model explainability, which is important for enhancing users’ trust in DL models by understanding the models’ vulnerabilities as well as identifying novel pathophysiological mechanisms linking the input and output datasets. However, recent studies have aimed to improve the explainability of DL models by creating novel methods to identify image and signal features that have the highest impact on the DL models’ decisions.30
Prediction of Ventricular Arrhythmia and Sudden Cardiac Arrest
Broad Prediction Algorithms
There are emerging published data on the utility of AI techniques for the long-term prediction of ventricular arrhythmia and SCA (months–years before the event), using various input data and ML algorithms. However, most published studies are hampered by low event numbers, which may be a significant source of overfitting and poor generalisability despite good internal validation. The majority of studies also focused on patients with severe left ventricle dysfunction and/or an ICD, who are likely to be a poor surrogate for individuals who experience SCA in the general population.5 For example, appropriate ICD shocks may not be effective surrogates given that a significant proportion of ICD-treated ventricular arrhythmias can spontaneously terminate without ICD intervention. Additionally, patients with primary prevention ICD mostly have LVEF <35%, and findings are not generalisable to the majority of SCA cases that occur in the general population (LVEF >35%). However, several published studies have reported findings that represent proof of concept and pave the way for further investigation.
A recent study by Rogers et al. estimated the cellular phenotypes of 42 CAD patients with EF ≤40% by measuring their monophasic action potentials during steady-state pacing in the electrophysiology laboratory.31 They trained and tested an ML model that achieved a sensitivity of 85% and a specificity of 86% in predicting ventricular arrhythmia in 3 years of follow-up. Interestingly, those with recurrent ventricular arrhythmia had prolonged duration and augmented height of the phase II plateau, and action potential simulation suggested that this was due to increased L-type calcium current or enhanced sodium–calcium exchange. This study design is an interesting proof of concept. Given that the sample size is relatively small and included only 13 patients with sustained ventricular arrhythmia, it would be worth evaluating in a larger number of patients.
Cardiac MRI-based Models
Another study by Popescu et al. developed a DL model to predict SCD in 10 years using cardiac MRI images and clinical covariates in 156 patients (41 events) with ischaemic heart disease.32 The model was externally validated in 113 patients (22 events) and achieved an AUC (area under the receiver operator characteristic curve) of 0.87 and 0.72 and 10-year integrated Brier scores of 0.12 and 0.14 for internal and external datasets, respectively. That study used an interesting approach of combining cardiac images with clinical covariates to predict SCD. Given that the number of SCD events is relatively low, further evaluation in additional larger sample sizes is warranted.
Other studies have also developed and trained cardiac MRI-based ML models to predict recurrent ICD therapies and SCA in the long term. Okada et al. used a substrate spatial complexity profile that was derived from gadolinium-enhanced cardiac MRIs to train and test an ML model that would predict appropriate ICD firings and SCD at 5 years in 122 patients with ischaemic cardiomyopathy and EF <35% (40 events). Their model achieved a moderate AUC of 0.72.33 Somewhat similarly, another study used a DL-derived cine risk score from cardiac MRI images that achieved an AUC of 0.69 for predicting appropriate ICD therapy in 7.1 years in 350 ICD recipients (96 events).34
Other Clinical Phenotyping Models
A recent study reported that an ML model using cardiac sympathetic function assessed with 123I-metaiodobenzylguanidine single-photon emission CT and clinical characteristics (e.g. age, sex, EF, New York Heart Association class) was able to separately predict arrhythmic events and heart failure death in 526 patients with chronic heart failure.35 Another study used demographics combined with clinical characteristics (including laboratory values and cardiac image indices) of 382 ICD recipients (with EF ≤35%) to predict appropriate ICD therapy or SCD during a follow-up time of 5.9 ± 2.3 years. Their ML model achieved an AUC of 0.88 and outperformed the Seattle Proportional Risk Model (AUC of 0.57).36 However, these latter two studies did not use independent datasets for external validation. Given that patients with recurrent ventricular arrhythmia and/or ICD shocks may need to undergo catheter ablation, models predicting recurrent ventricular tachycardia after ablation could potentially support clinical decision-making in the future.37
In summary, these studies have trained and tested AI models to predict ventricular arrhythmia and/or SCA in the long term with moderate–good accuracy and represent an initial step. For reference, previous studies have estimated that the AUC for LVEF in the long-term prediction of SCD is only 0.59–0.68 in various populations, and a recent study demonstrated that this can be improved with the addition of AI-based ECG analysis.38–40 The current literature suggests that AI algorithms have the potential to improve long-term ventricular arrhythmia and SCA risk stratification. However, additional studies with larger sample sizes, external validation, more diverse patient samples and carefully adjudicated endpoints will be needed to assess the clinical utility of ML models in improving long-term ventricular arrhythmia and SCA prediction (Figure 1).
Prediction in Specific Ventricular Arrhythmia/Sudden Death Syndromes
The vast majority of all SCAs in middle-aged and older individuals occur in association with CAD.24,41 In younger individuals (<35 years) there is a higher likelihood of detecting non-ischemic cardiac conditions such as hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), long QT syndrome (LQTS) and Brugada syndrome (BrS).42 These patients have different clinical characteristics and cardiac phenotypes from CAD patients, and hence it is important to have a specific focus on developing distinct risk stratification tools for patients with non-ischaemic arrhythmogenic cardiomyopathies.
Smole et al. used multiple variables (e.g. demographics, physical examination, genetics, imaging, medications) in 2,302 HCM patients to train an ML model to predict the 5-year risk of SCD.43 Their model achieved an AUC of 0.70 for SCD and outperformed a previously established conventional SCD risk calculator for HCM patients (AUC of 0.63).44 Two other studies aimed to develop and train ML models in the identification of HCM patients at high risk of ventricular tachycardia and VF.45,46 Another study used cardiac MRI features as model input data and achieved an AUC of 0.91, while yet another used 22 clinical covariates (including clinical history, cardiac imaging, and medication) and achieved an AUC of 0.83.45,46 Additionally, Lyon et al. used an unsupervised ML approach to identify high-risk ECG phenotypes in HCM patients and found that primary T wave inversion with normal QRS was associated with the highest SCD risk score.47 Relatively small studies on BrS, LQTS, DCM and tetralogy of Fallot have also demonstrated that ML models may be useful and can outperform conventional statistical methods to predict ventricular arrhythmia and SCD.48–52
Although these models use diverse study designs and input data, these are universally trained and tested on small numbers of endpoints and events (<100), which is a potential limitation for model generalisability. Therefore, significantly larger sample sizes would be ideal for future studies. Compared with complex traits such as coronary disease and heart failure, inherited ventricular arrhythmia and SCA syndromes are more homogeneous but the incidence in the overall population is low. Acquired cardiac diseases have a more complex phenotype consisting of multiple heterogeneous clinical conditions, and thereby create a different kind of challenge for model generalisability.
Prediction of Imminent Ventricular Arrhythmia and Sudden Cardiac Arrest
Broad Prediction Algorithms
Hospital Setting
In-hospital cardiac arrest (IHCA) is a fatal event in the majority of patients. The incidence is dependent on the study population and is estimated to be 1–17 events per 1,000 hospital admissions with a higher than 70% mortality rate.2 In most of the cases, the patients have abnormal vital signs during the preceding 4 hours.53 The total number of annual IHCAs is lower than out-of-hospital cardiac arrest (OHCA) (~290,000 versus ~360,000 in the US, respectively). Compared with OHCA, IHCA is considered to be a distinct event with unique opportunities and challenges and a higher likelihood of non-cardiac aetiologies.2 Hence, ML models that predict IHCA may not apply to the prediction of OHCA.
One of the leading causes of hospitalisation in the US is heart failure (HF), which accounts for approximately 1 million hospital admissions each year.54 A recent study focused on hospitalised HF patients and used demographics, medical history data, laboratory values, physiological measurements and medication in 2,794 hospitalised HF patients. The authors trained and tested an ML model that predicts malignant arrhythmias (117 events) during the hospital stay with an AUC of 0.867.55
Kwon et al. developed a DL-based early warning score, which included four basic vital signs (systolic blood pressure, heart rate, respiratory rate, and body temperature).56 Their study sample consisted of 52,131 patients admitted to two hospitals (419 IHCAs), and their model achieved an AUC of 0.850, which significantly outperformed the modified early warning score (MEWS) (AUC of 0.603). Later, the authors extended this DL model by adding diastolic blood pressure, age and the recorded time of each vital sign in 173,368 hospitalised patients from general wards of five hospitals (224 events), and divided the data into internal and external datasets. This model also had a performance that was superior to MEWS in both the internal (AUC: 0.860 versus 0.754, respectively) and external (AUC: 0.905 versus 0.785, respectively) datasets.57 Although the number of IHCAs remains relatively small, other slightly smaller studies in hospitalised patients have also suggested that ML models can achieve good discriminative values and outperform conventional prediction models using clinical record data, vital signs and laboratory values.58–62
Emergency Department Setting
In addition to hospitalised patients, ML models may assist with the triage of patients in emergency departments (EDs), given that high-risk patients may require early coronary intervention or intensive cardiac monitoring. A study by Ong et al. used heart rate variability parameters and vital signs to train and test an ML model for the prediction of cardiac arrest within 72 hours in 925 ED patients (43 events).63 Their model achieved an AUC of 0.78, which outperformed the conventional MEWS score (AUC of 0.68). That study had a small number of endpoints and no external validation, but subsequent studies have also suggested that relatively simple ML models based on vital signs can outperform MEWS in ED patients.64–66 However, in addition to the lack of external validation, one limitation of these papers is the diversity of clinical conditions in the ED patients. To overcome this, Wu et al. used 20 clinical features from 166 acute coronary syndrome (ACS) patients with IHCA and 521 ACS controls to develop various ML algorithms that predict cardiac arrest within 24 hours. Their best model achieved a promising AUC of 0.958, which outperformed commonly used risk prediction models such as GRACE, NEWS and MEWS (AUCs 0.67–0.73).67 However, this model was not externally validated.
Although simple vital signs can be used to train an ML model with a good performance, a multimodal AI approach combining vital signs with images or ECG waveforms could provide additional accuracy to unimodal ML models. The potential for using ECG waveforms in predicting IHCA within 24 hours was demonstrated by a recent study that used demographics and ECGs of 25,672 patients who were admitted to two hospitals.68
Prediction of Specific Ventricular Arrhythmia and Sudden Death Syndromes
While the aforementioned short-term prediction models are trained and tested on broad patient groups, a specific focus on high-risk conditions is also warranted. A few studies have trained and tested ML models in ICD patients, and achieved good accuracy: Shakibfar et al. used nine ICD variables from 19,935 ICD patients to predict electrical storm (ES) within 1 day (2,367 ES events occurred in 1,410 patients).69 Their ML model achieved an AUC of 0.80 and the most relevant variables were the percentage of ventricular pacing and daytime activity. Another study used heart rate variability data from 788 ICD patients (from the SCD-HeFT trial) to predict ICD shocks within 10 seconds and 5 minutes (6,660 regular rhythms and 230 pre-shock rhythms).70 Their ML model reached a good AUC of 0.81 for a 5-minute prediction and an AUC of 0.87 for a 10-second prediction of shock. Predicting an impending ICD shock may be important in alerting healthcare providers as well as guiding patients in the avoidance of situations in which an ICD shock may cause substantial harm (e.g. driving, risk of falling).
Patients undergoing dialysis have been recognised to be at a significantly increased risk of SCA.71 However, there is still a significant gap in the knowledge of risk factors and mechanisms of haemodialysis-related SCA, and no accurate risk prediction models exist. Goldstein et al. used comprehensive EHR data (demographics, dialysis-specific factors, laboratory values, physiological measurements, medications) from a large sample of dialysis sessions (22 million sessions, 1,697 SCDs) to train and test an ML model to predict SCD within 1 day of a dialysis session. Their model achieved a good performance (AUC of 0.799), which illustrates the potential of using large EHR data to develop short-term risk prediction models for relatively rare events.72
The Future of AI for Ventricular Arrhythmia and SCD Prediction
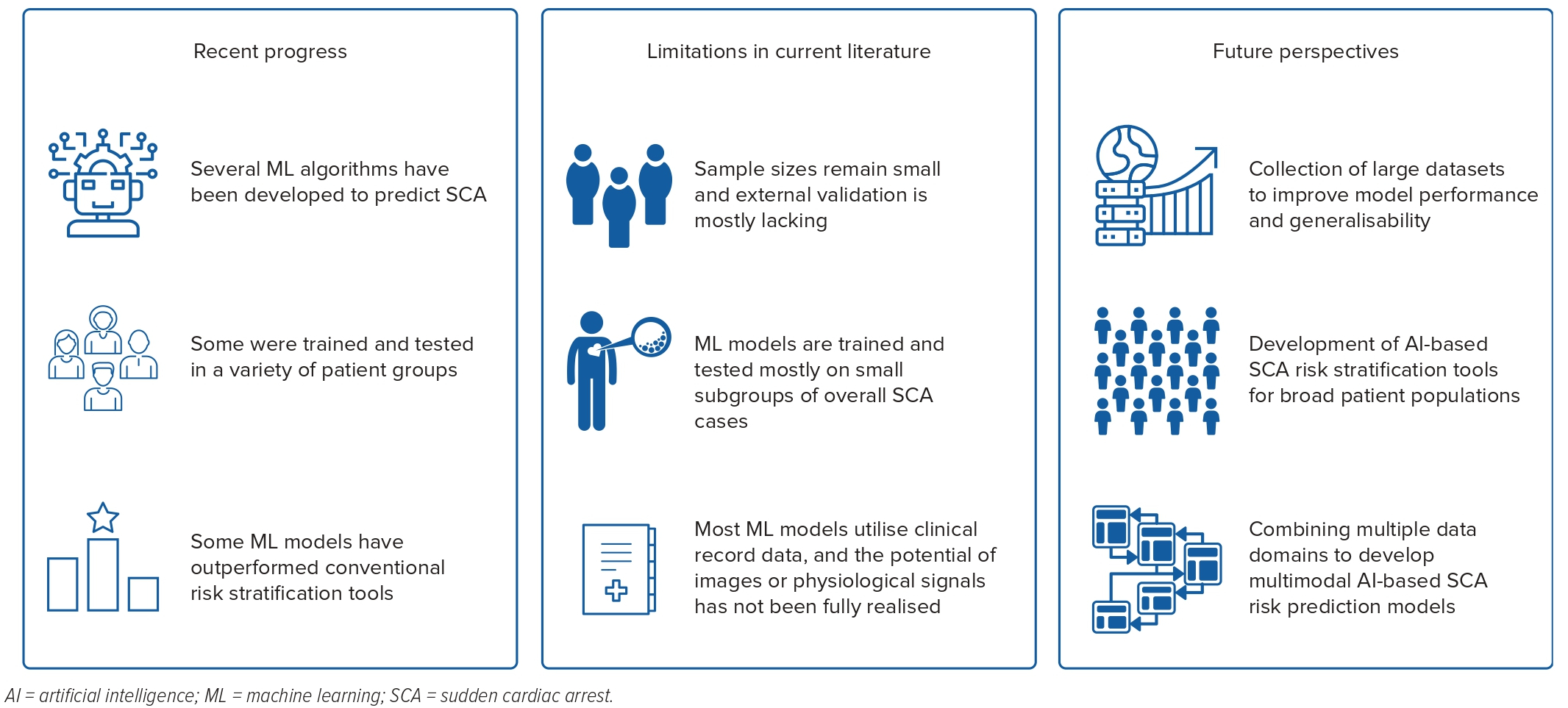
Published studies have demonstrated proof of concept regarding the utility of ML models for the detection of individuals who are at high risk of ventricular arrhythmia and SCA in the short and long term. However, some limitations and knowledge gaps should be addressed in future studies.
Although most ML models in previous studies have shown good discriminative value in internal validation, small sample sizes and the lack of external validation tend to reduce the generalisability of these models. In small sample sizes, ML models are prone to overfitting, which may lead to poor performance in independent and heterogeneous datasets. Moreover, while previous studies have successfully developed ML models for specific SCA risk groups (e.g. patients with ICD, severely reduced EF, HCM, LQTS, BrS), future studies are needed to develop models that can be applied to predict SCA in broader groups. Although severely reduced EF and rare SCA syndromes are important risk factors for SCA, most of the cases occur in subjects without these conditions.5
The current literature lacks ML models that would predict pre-hospital SCA in the near term. Although previous studies have demonstrated that ML models using simple vital signs have the potential to outperform conventional risk stratification tools in predicting IHCA, it is important to recognise that IHCA is considered to be a separate entity from OHCA and that these short-term models are developed with considerably different settings compared with long-term models of out-of-hospital ventricular arrhythmia and SCA.2 These models may not apply to out-of-hospital settings. Given that a significant proportion of SCAs is preceded by warning symptoms and that there are no broad short-term prediction models for pre-hospital OHCA, there is room to improve short-term SCA prediction (Figure 2).
Accurate SCA prediction will be likely to require a combination of biomarkers. However, previously established SCA risk factors are usually not specific to SCA but instead predict non-sudden cardiac death as well.23 This is an important and sometimes overlooked limitation in SCA risk calculators. The benefit from preventive treatment with an ICD is not only dependent on the absolute SCA risk but also on the relative sudden versus non-sudden death risk.73–75 Competing modes of death complicate accurate SCA risk stratification, and this aspect becomes even more relevant due to ageing of the general population and the increasing mean age of SCA patients. There is room for AI-guided identification of patients with a high proportional SCA risk.39
The creation of SCA prediction models requires careful adjudication of the cause and mechanism of the event. This includes the exclusion of non-cardiac acute events that may also manifest as cardiac arrest and sudden death (e.g. pulmonary embolism, aortic dissection, stroke). Furthermore, classification of SCA events based on underlying mechanisms into shockable and non-shockable SCAs has important clinical implications. Given that the proportion of non-shockable SCAs has significantly increased in recent decades, and that the long-term prevention of SCA is mostly based on ICD implantation and terminating shockable rhythms, a renewed focus on predicting specifically shockable SCAs is warranted.38 Given that most of the SCAs manifest with non-shockable presenting rhythm, achieving good prediction accuracy for overall SCA does not necessarily mean that these high-risk patients would benefit from primary prevention ICD implantation.
Most published AI models use tabular EHR data (e.g. demographics, clinical variables), and the potential of simultaneously using raw image and physiological signal data has not been realised. For example, several ECG variables and conventional ECG-based risk scores have been associated with an increased risk of SCA in the long term, but the usefulness of the ECG waveform-based DL model is yet to be investigated.76–78 Current AI models mostly use unimodal input data. However, in the future, multimodal and explainable AI are likely to unlock many opportunities in the field of ventricular arrhythmia and SCA prediction by using and integrating multidimensional patient data such as by combining EHR data with ECG, other signals, and imaging modalities as well as genomic and proteomic information (Figure 3).22 
Clinical Perspective
- Lethal ventricular arrhythmias resulting in sudden cardiac death (SCD) is a major cause of mortality worldwide.
- Conventional SCD risk stratification tools are increasingly recognised as being inadequate.
- Recently published studies have demonstrated the potential of machine learning models to improve the detection of individuals at high risk of SCD.
- Some limitations in methodology need to be overcome so that important knowledge gaps in the field could benefit from deployment of artificial intelligence tools for long-term as well as near-term prediction and prevention of SCD.
- Future studies will benefit from the usage of unimodal, multimodal and explainable artificial intelligence in large databases with consistent definitions of SCD.










