Vasovagal syncope (VVS) is one of the most common types of syncope. By the age 60 years, 42% of women and 32% of men already had at least one episode of syncope.1 Although the outcome of VVS is generally benign, it can result in injury and it can affect quality of life.2,3 The underlying pathophysiology of VVS results from a reflex causing hypotension and bradycardia, which is mediated through excessive activation of vagal activity.4,5 Results of traditional treatments, including increased salt and water intake, physical counterpressure manoeuvres, drug therapy with fludrocortisone or midodrine, have been disappointing.5
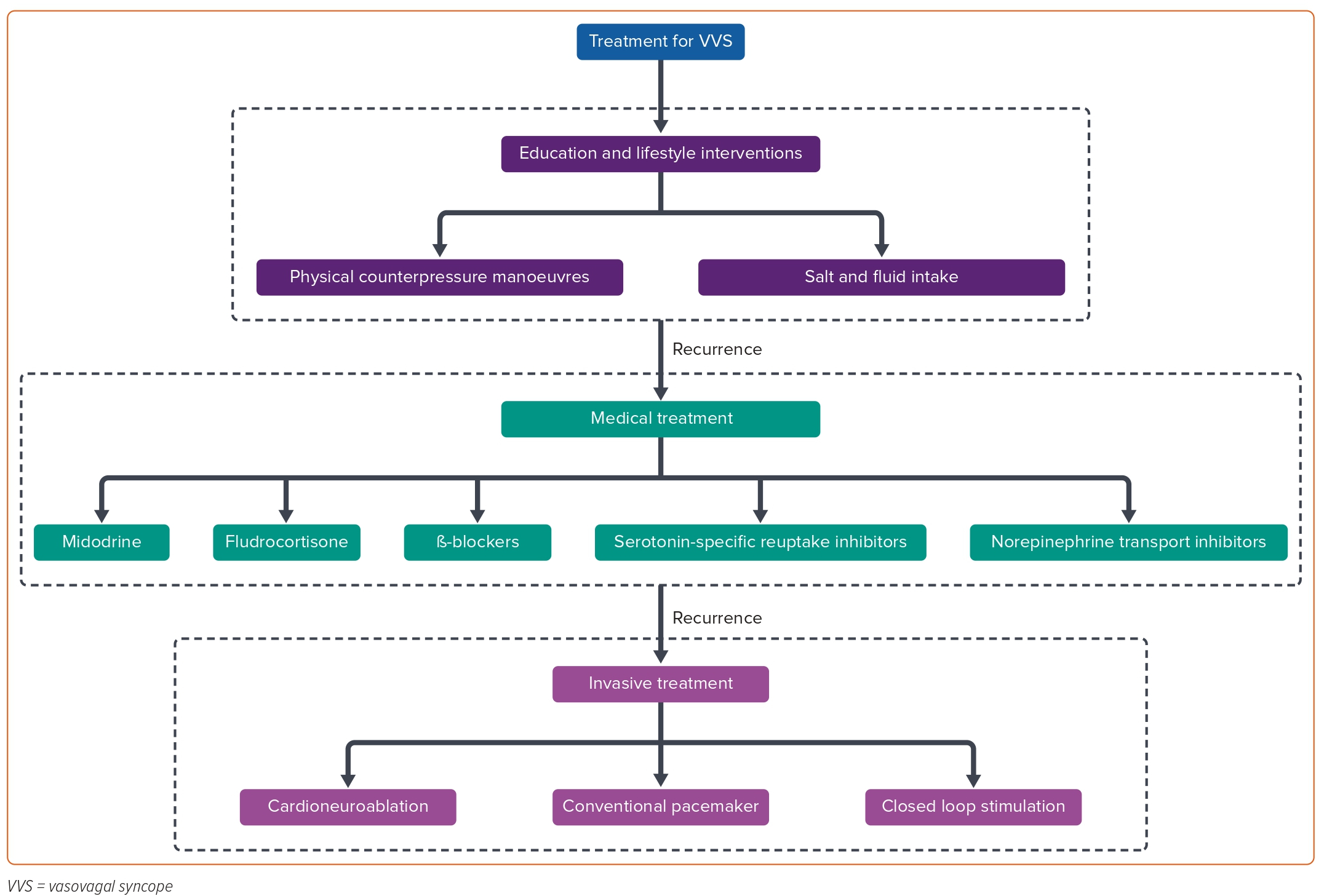
Previous studies demonstrated the importance of autonomic activity in AF. Ablation of AF-nests, the tissue with a complex frequency spectrum in sinus rhythm which is highly associated with vagal activity, has been used to treat AF.6 In the past two decades, cardioneuroablation (CNA) has been employed to treat functional bradyarrhythmia and VVS by ablating the neuromyocardial interface on the endocardium and the ganglionated plexi (GP), intrinsic structures located in the epicardial atrial fat pads. CNA can inhibit excessive excitation of vagal activity and rebalance the autonomic nervous system.7–9 Figure 1 illustrates current approaches to treating VVS.
Preliminary data from CNA in treating selected patients with VVS are encouraging, however, some unfathomed issues such as patient selection, ablation strategies, limit its applicability.10–12 In this review, we described the techniques of CNA for VVS, summarise the key developments and discuss future directions.
Rationale of Cardioneuroablation for Vasovagal Syncope
Pathophysiology of Vasovagal Syncope
Although novel therapies are constantly emerging, the pathophysiology of VVS remains a matter of debate. A widely accepted theory is that VVS is caused by an abnormal autonomic reflex. The baroreflex mediated by the autonomic nervous system (ANS) is constitutively active to maintain the homeostatic status. For instance, rising to an upright position leads to pooling of 500–800 ml of blood in the peripheral circulation, which activates sympathetic nerves to prevent hypotension.13 During a VVS episode, an abnormal reflex response causes venous pooling in the periphery, leading to excessive sympathetic outflow and an increase in ventricular contraction which activates sensory receptors located in the inferoposterior portions of the left ventricle when wall tension changes, paradoxically increasing neural traffic to the central nervous system.14 Eventually, it causes a persistent rise in vagal activity, followed by bradycardia and loss of consciousness.
Rationale of Cardioneuroablation
Parasympathetic hyperactivity is a fundamental mechanism of VVS, making it a possible target to treat VVS with cardioinhibition. The autonomic activity of the heart is regulated by several levels of feedback loops between the heart and the peripheral and central nervous systems. The cardiac ANS can be divided into the extrinsic and intrinsic components according to the location of postganglionic neurons that provide fibres from the ganglion to the effector organ.15,16 Most of the neurons of the intrinsic cardiac ANS converge at several GP within the epicardial fat pads. The intrinsic cardiac ANS includes efferent parasympathetic and sympathetic nerves, afferent neurons and local circuit neurons/intermediate neurons. The postganglionic intrinsic nerves then extend to specific atrial or ventricular regions, such as the sinoatrial node, atrioventricular (AV) node and the roots of pulmonary veins.17 GP integrate preganglionic and postganglionic nerve fibres to affect heart rate and cardiac function. By targeting the GP with catheter ablation, CNA has emerged as a novel and effective therapy for VVS by ablating the endocardial neuromyocardial interface and GP.7
Pivotal Trials of Cardioneuroablation in Vasovagal Syncope
Since the average atrial wall thickness is approximately 3 mm, radiofrequency energy can be transmitted through the atrial wall to the epicardial GP, which enables the possibility of GP ablation via the endocardium. In 2005, Pachon et al. proposed a novel technology, named cardioneuroablation, for the management of patients with dominantly excessive vagal outflow.7 The study included five cases with VVS, seven patients with functional high degree AV block and 13 patients with sinus node dysfunction. The mean follow-up period was 9.2 months; favourable results were shown in all cases with relief of symptoms. It is noteworthy that Pachon et al. developed a novel method to locate the entry of vagal fibres into the atrial myocardium by spectral analysis, but the requirement of proprietary pre-amplifier and spectral analysis software limited its application. They later simplified the spectral analysis to identify not only the major GP but the many micro-GP without a special pre-amplifier and obtained favourable results.18
To further overcome the main challenge of locating GP, Scanavacca et al. delivered high-frequency stimulation (HFS) to localise the GP in a patient with frequent episodes of VVS.19 We further demonstrated the feasibility and efficacy of HFS-guided CNA in a series of cases.8 Contrary to the classical biatrial ablation method reported by Pachon et al., we defined a new technique via catheter ablation of GP only in the left atrium (LA) based on linear ablation of AF in which the vagal reflex (VR) was frequently observed.7,8 We also reported the long-term efficacy of GP ablation in the LA in a larger sample size.10 CNA was performed in 57 patients with VVS. During the mean follow-up of 36 months, 52 patients (91.2%) remained free from syncope. The outcome was not different between the HFS-guided approach and an anatomical approach for syncope (100% versus 89.4%, p=0.348) or recurrent prodromes (50% versus 76.6%, p=0.167). Debruyne et al. proposed a less extensive and more specific approach for CNA, which was based on partial ablation of the right anterior GP (RAGP) of the right atrium (RA).20 The right-side ablation strategy without the need for transseptal puncture appeared to provide a comparable therapeutic effect. The importance of RAGP was further confirmed in our study.11 Additionally, extending the RAGP ablation to the left interatrial septum seems to be crucial to attenuate the vagal tone of the sinus node.7,11,18 However, in a recently published meta-analysis of CNA, RA ablation was associated with a significantly lower freedom from syncope compared with LA ablation only and biatrial ablation.21 A randomised controlled trial (RCT) comparing the effect of RA ablation only and LA ablation only may help to resolve the disagreement.
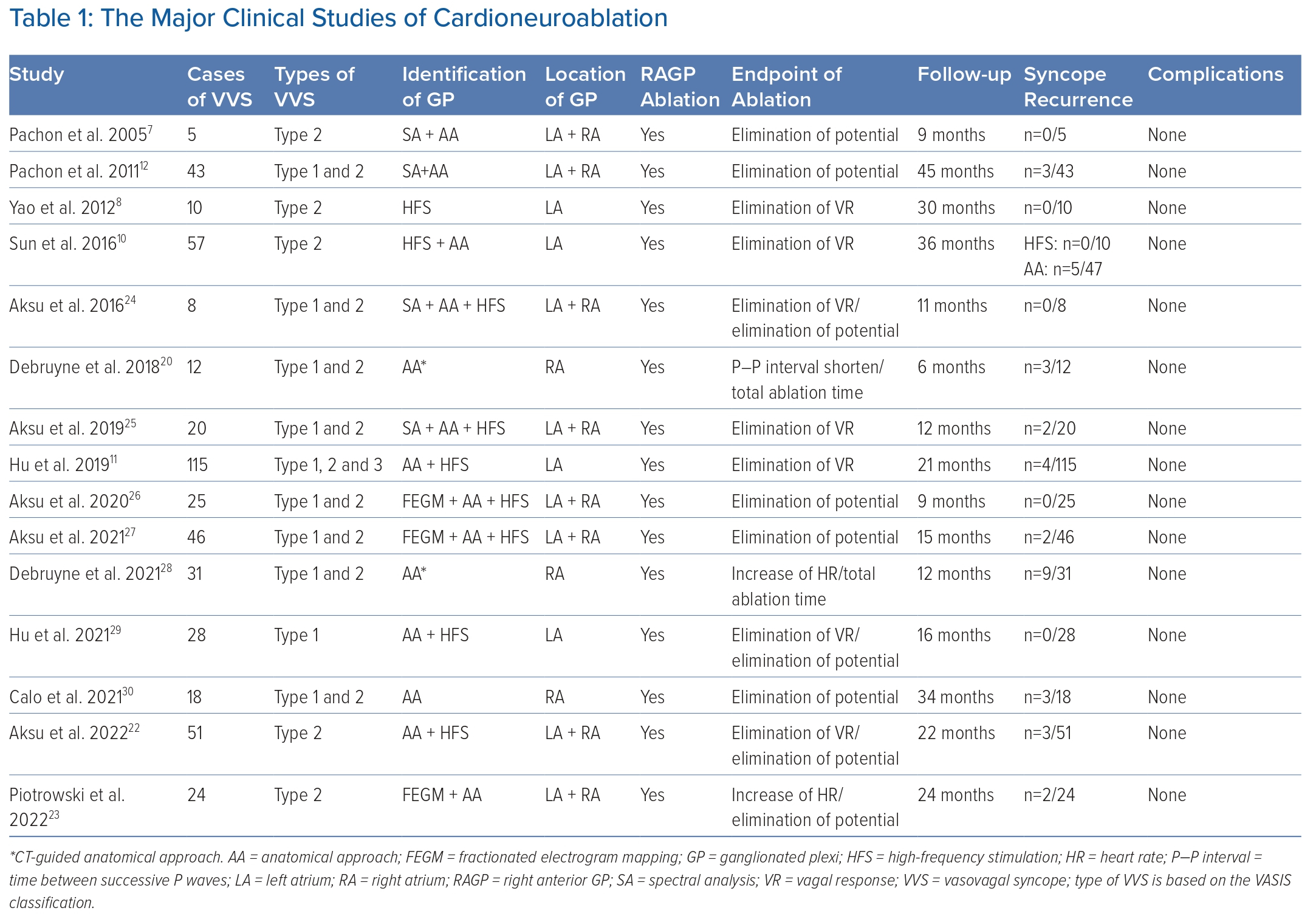
A case-control study by Aksu et al. investigated the long-term effect of CNA compared with conservative treatment in patients with VVS.22 After a mean follow-up of 22 months, CNA led to a marked decreased risk of syncope recurrence (HR 0.23; 95% CI [0.03–0.99]; p=0.049). Recently, Piotrowski et al. reported results of the first RCT of CNA for VVS.23 In the study, patients with cardioinhibitory VVS were randomly divided into the CNA group (n=24) and the optimal nonpharmacological therapy group (n=24). After 2 years follow-up, syncope recurrence in the CNA group was significantly lower than the control group (8% versus 54%; p=0.0004), which provided more reliable evidence. Other pivotal studies of VVS are summarised in Table 1.12,24–30
The placebo effect is also a critical issue when considering the efficacy of GP ablation. In previous studies on pacing to treat VVS, there was a huge placebo effect.31,32 Sud et al. reported that there was a significant ‘expectation effect’ of pacing in VVS with patients showing a reduced risk of recurrent syncope (OR 0.16; 95% CI [0.06–0.40]; p<0.001) simply by knowing that a permanent pacemaker was implanted and functional.33 Of note, RCTs comparing CNA with other treatments including lifestyle interventions and drugs cannot eliminate the placebo effect. The results of non-randomised, unblinded trials should be interpreted with caution. Therefore, sham-controlled, randomised clinical trials, which could assess the true effect of CNA, are needed before CNA is considered as standard care for highly symptomatic VVS.
Current Status and Unanswered Questions
Patient Selection
The head-up tilt test (HUTT) may help determine whether patients have the autonomic substrate for VVS. A positive response is defined as a clinically reminiscent presyncope or syncope associated with hypotension and usually bradycardia.5 Accordingly, HUTT is frequently employed to diagnose VVS. The VVS classification goes, according to the HUTT response: type 1 (mixed response); type 2A (cardioinhibitory without asystole); type 2B (cardioinhibitory with asystole >3 second); and type 3 (pure vasodepressor response).34 Considering CNA does not directly affect the autonomic nerve on blood vessels, most previous studies excluded vasodepressive VVS for ablation.12,20,35 Therefore, the efficacy of CNA for vasodepressive VVS is yet to be elucidated. However, Hu et al. were the first to study CNA in this condition showing that there may be a good response even in the vasodepressor-type VVS.11
Although HUTT is a classical non-invasive method to induce syncope reproduction, the poor reproducibility of HUTT significantly limits its diagnostic value.36,37 Specifically, the reproducibility of the same response in a second test when the first was positive ranged from 31–92% in different studies.38 Previous studies suggested that the vasovagal reflex was often triggered by adjunctive agents, such as isoproterenol, nitrates and clomipramine; however, increasingly aggressive protocols decrease specificity.5 Heart rate variability (HRV) derived from an analysis of the RR interval of ECG is a valuable non-invasive test to assess the ANS function.39 Pachon et al. reported that all autonomic parameters of time- and frequency-domain HRV were decreased at 2 years post-CNA, demonstrating the long-term efficacy of CNA and the value of HRV in evaluating the ANS function.35 However, conflicting results question the diagnostic value of HRV for VVS.40,41 Cardiac deceleration capacity (DC) is derived from analysis for HRV and is introduced to quantitatively assess the cardiac vagal function.42 A reduction of cardiac DC reflects a decrease in the vagal tone of the cardiac autonomic function.43 Our previous study has demonstrated that DC >7.5 ms may serve as a good tool to monitor cardiac vagal activity and discriminate VVS by the area under the receiver operating characteristic curve (AUROC) of 0.809, particularly in those with negative HUTT.44 We have found that DC could be applied to guide CNA in VVS, and a night-time baseline DC of ≥10 ms may act as an indication for CNA in patients with VVS.45 In addition, an atropine test with a sinus heart rate increase of ≥25% or ≥90 bpm with 0.04 mg/kg IV atropine sulfate has been used to select patients suitable for CNA.46
To summarise, although the indications of CNA for VVS are yet to be determined, HUTT and DC may serve as non-invasive tests to diagnose and classify VVS. As a novel approach to reflect vagal function with certain specificity, it is promising to use DC to select patients suitable for CNA.
Identification of Ganglionated Plexi
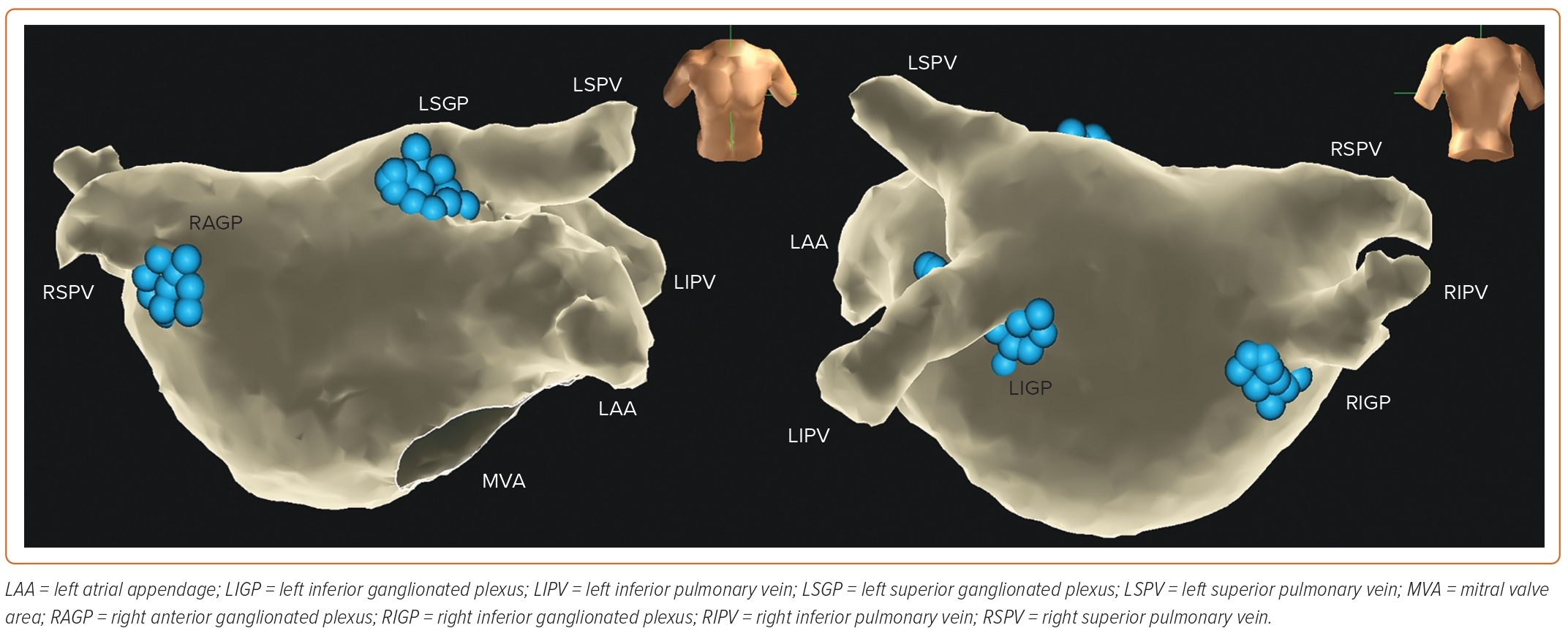
According to the anatomical location, GP can be divided into five atrial and five ventricular GP; the latter do not affect the efficacy of CNA. For ablation, the three left atrial GP (LAGP) are further divided into five plexus subdivisions: left superior GP (LSGP; located in the superolateral area around the root of the left superior pulmonary vein), left inferior GP (LIGP; located in the inferoposterior area around the root of the left inferior pulmonary vein), right inferior GP (RIGP; located in the inferoposterior area around the root of the right inferior pulmonary vein), right anterior GP (RAGP; located in the superior-anterior area around the root of the right superior pulmonary vein), and left lateral GP (LLGP; located in the area around the ligament of Marshall) (Figure 2).47 In addition, the vein of Marshall also belongs to the intrinsic cardiac ANS and parasympathetic fibres from the vein of Marshall innervate the surrounding LA structures and the coronary sinus. Moreover, investigators have found that ablating the GP located in the interatrial septum increased the heart rate.18
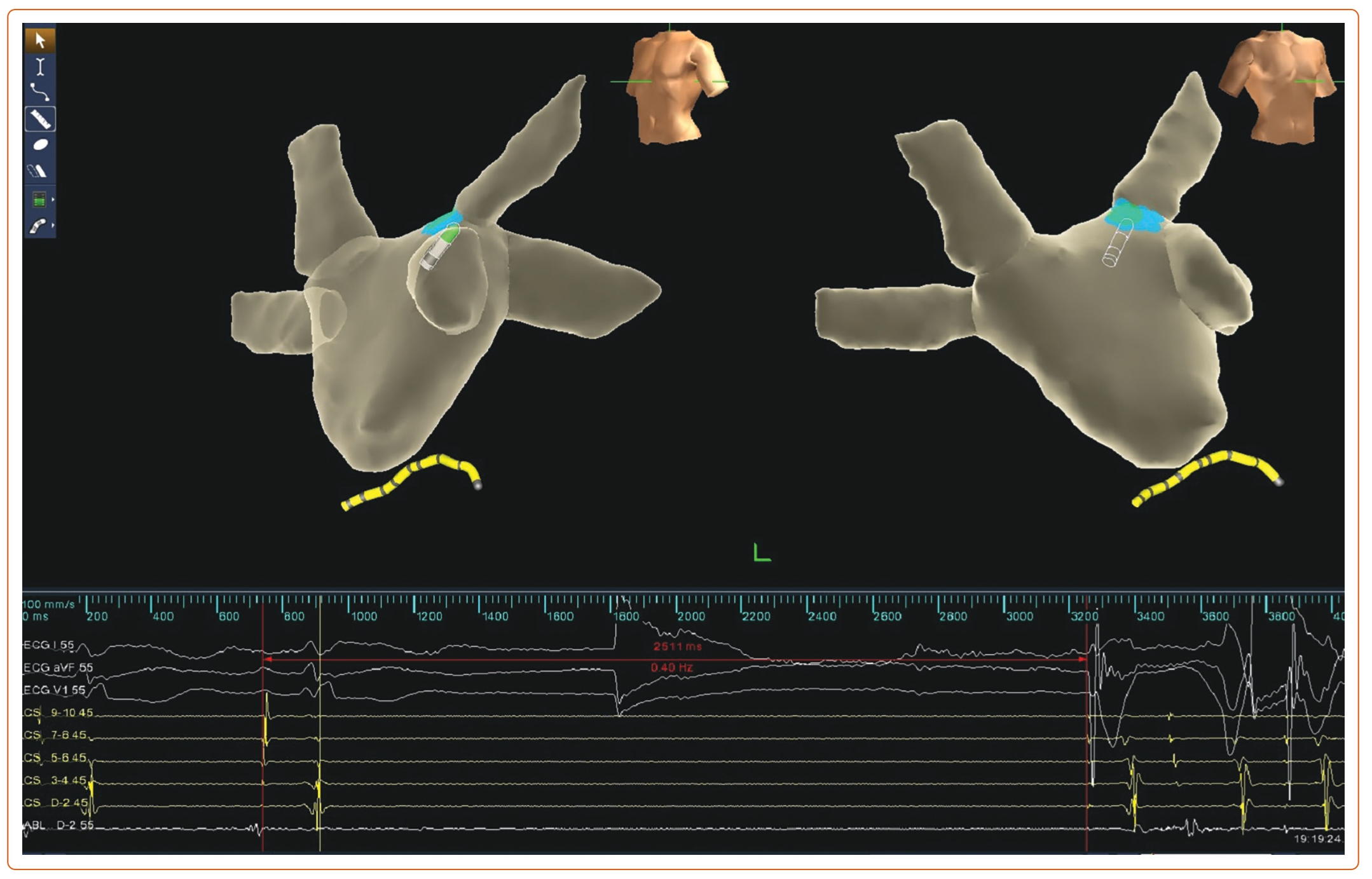
There are three main approaches to performing GP ablation: HFS-guided, anatomy-guided and spectral mapping/fractionation-guided approaches. HFS was initially designed to identify GP location during circumferential pulmonary vein isolation for AF.48 HFS is performed on each presumptive GP site. During the HFS period, a positive VR is defined as transient ventricular asystole, AV block, or an increase in the mean RR interval of 50% (Figure 3).49 Moreover, the specific anatomical distribution of GP enables CNA with the anatomical approach. The endpoint of the ablation procedure is the elimination of all VR at each identified target in both approaches. However, HFS cannot identify smaller GP and there is a large variation in GP location. Moreover, the positive rate of HFS response is relatively low, which may limit the application of HFS, although the anatomical approach and HFS-guided approach produced similar outcomes.10
The spectral analysis can also be used to localise GP based on different spectrums resulting from autonomic innervation.7 In recent years, Pachon et al. proposed the ‘fractionation software’ based on the theory of AF-nests, by setting the high pass and low pass filters to 200 and 500 Hz respectively. The ganglia sites were defined as those with the fractionated multicomponent signal with ≥4 deflections in this software.18 This simplified fractionation approach without the need for a specialised spectrometer could identify the great number of micro-GP that exist beyond the conventional GP. In addition, Aksu et al. used the fractionated electrogram (FEGM) mapping based on the EnSite Precision system (Abbott Vascular).50 FEGM-guided CNA without the need for the use of additional equipment decreases procedure and fluoroscopy times and could accurately identify GP targets. In a meta-analysis, Vandenberk et al. found that the above techniques used to identify GP did not show any significant difference in freedom from syncope.21
Ablation of Ganglionated Plexi
All CNA approaches should be conducted via a 3D navigation system. Pachon et al. reported that thermo-controlled radiofrequency should be limited to 50W/60°C (non-irrigated) and 30W/45°C (irrigated).12 In our early studies, we used the non-irrigated catheters with a limited set of 60W/60°C and delivered at least 30 seconds to achieve VR in each GP site.8,10 While non-irrigated catheters were used in earlier studies, irrigated catheters are recommended for CNA to improve safety and efficacy.
Considering the complex integration among GP, the sequence of GP ablation may affect the ablation response.51,52 In previous studies, we observed an immediate increase in heart rate during catheter ablation of the RAGP that is not observed during ablation of other GP in the LA.10,11 Recently, we performed a prospective, randomised study to clarify the effect of different sequences of GP ablation. A total of 28 VVS patients were randomly assigned to two groups according to different orders of GP ablation: group A: LSGP – LIGP – RIGP – RAGP; group B: RAGP – LSGP –LIGP – RIGP. The results showed that the sequences with RAGP ablated first effectively inhibited the VR during ablation of other GP of the LA.29 In addition, we reported that RAGP ablation could significantly inhibit VR during pulmonary vein isolation in paroxysmal AF, suggesting the essential effect of RAGP in modulating ANS function.53 Accordingly, we hypothesised that RAGP could be the primary target of CNA. The importance of RAGP in regulating ANS function has been previously demonstrated. Hou et al. suggested that RAGP served as the final common pathway for autonomic neural inputs to the sinoatrial node.49,54 Wang et al. reported that selective ablation of RAGP would successfully attenuate the baroreflex, further demonstrating the unique effect of RAGP.55 CardNMH3 (NCT04755101) is a multicentre, double-blind, randomised trial with a sham control group investigating the efficacy and safety of CT-guided RAGP ablation only to prevent recurrence of syncope in VVS which verify the critical role of the RAGP in treating VVS. To date, the best strategy for CNA is still unknown. We adopted the LAGP as the primary ablation targets, whereas Debruyne et al. selected RAGP, and both approaches achieve similar success rates (>90%).8,20,28 Although Vandenberk et al. reported that RA ablation only may be inferior to LA ablation, this finding still needs to be demonstrated in the related RCTs.21
Ablation Endpoint of Cardioneuroablation
The efficacy of CNA may depend on the completeness of vagal denervation; however, there is no strategy to evaluate the completeness of vagal denervation. There have been multiple endpoints proposed in different studies. Elimination of positive VR assessed by HFS may be the suitable ablation endpoint. After CNA, HFS is repeated at each ablation site to evaluate the VR. Further ablation is performed when VR remained positive.8 However, HFS is neither sensitive nor specific and is unable to predict the long-term effect. Atropine response abolition is also employed to test the effect of CNA.7 Absence of heart rate response to atropine implies appropriate vagal denervation. Atropine infusion should only be done at the end of the procedure for its long-lasting effect. Pachon et al. developed another approach to evaluating vagal denervation.56 The great proximity of the vagus nerve to the internal jugular vein and the carotid artery enables extracardiac vagal stimulation (ECVS) through the internal jugular vein approach. A quadripolar electrode catheter is advanced to both jugular foramens where the electrode is close to the vagus nerve. VR was initiated by neurostimulation (pulse amplitude of 1 V/kg body weight up to 70 V, 50 ms width, 50 Hz frequency for 5 seconds). The typical observed response is asystole or AV block.18,56 Continued positive responses suggests incomplete vagal denervation. ECVS can be repeated during CNA to assess the completeness of vagal denervation. Piotrowski et al. optimised and visualised the ECVS, using ultrasound to localise the vagus nerve and the ideal position to position the catheter.57 The results showed that ultrasound-guided ECVS was feasible and VR was achieved more frequently than fluoroscopy-guided ECVS. ECVS is a promising method to evaluate vagal denervation, but long-term follow-up is needed to assess if it is superior to other approaches.
Long-term Effects of Cardioneuroablation
Considering CNA is a novel technology, many studies have been published with relatively short follow-up times. Pachon et al. reported that long-term vagal reflex attenuation can be achieved by CNA, with a mean follow-up of 45 months.12 However, CNA may destroy more autonomic nerves than neurons. The possibility of reinnervation exists. Previous studies have demonstrated that vagal reinnervation autonomically occurred within the first 6 months after GP ablation.58,59 On the contrary, Pachon et al. found no HRV parameters recovered 2 years after CNA, suggesting the long-term efficacy of CNA in VVS.35 Even after heart transplantation, vagal reinnervation was observed in long-term follow-up.60 Long-term outcomes of CNA are yet to be determined.
Safety of Cardioneuroablation
Scanavacca et al. reported acute occlusion of the sinus node artery after CNA procedures in two patients.61 The authors suggested operators take measures to prevent it, including evaluating the aortic angulation in older patients before the procedure. No more severe complications of CNA have been reported. We may conclude that CNA is a relatively safe procedure. As an invasive operation, there are some potential risks of CNA including vascular complications (AV fistula, pseudoaneurysm), pericardial effusion, thrombotic events, and so on. Future large-scale prospective registries are needed to further demonstrate its safety.
Other Indications for Cardioneuroablation
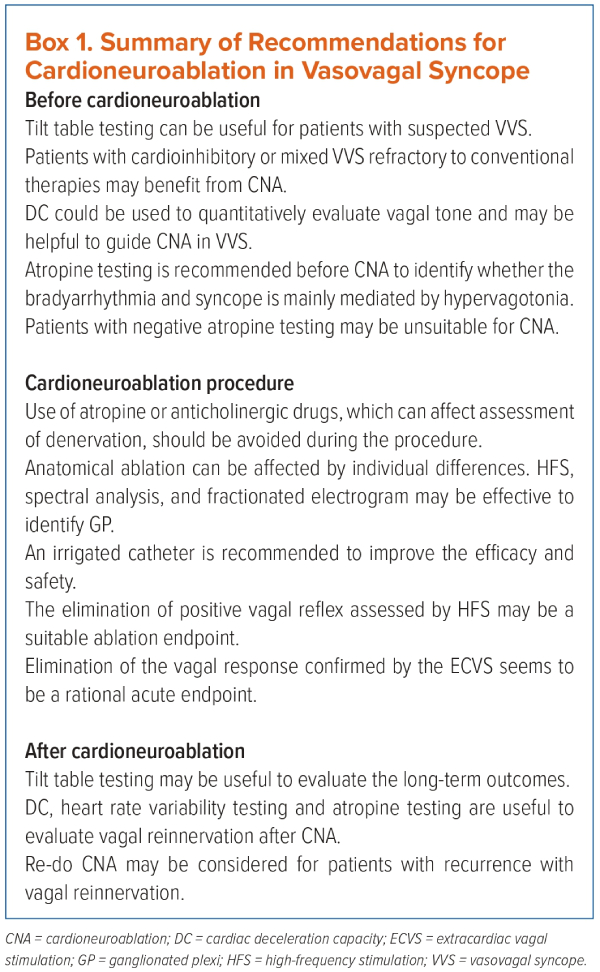
The efficacy and safety of CNA for treating VVS and sinus node dysfunction have been demonstrated in numerous studies. In this review, we summarised the recommendations of CNA for treating VVS (Box 1).
Recently, Aksu et al. reported encouraging medium-term outcomes for treating functional AV block using CNA.62 The favourable performance was also verified in long QT syndrome.63 In addition to bradyarrhythmia, CNA can also be employed as adjuvant therapy for AF.64–66 Neto et al. suggested that CNA could be an alternative treatment for bradyarrhythmia induced by overtraining.67 Similarly, CNA could be considered for cardioinhibitory carotid sinus syndrome.68 However, the specific mechanisms and long-term effects need to be clarified in future research.
Conclusion
CNA is a promising therapy to treat arrhythmias caused by excessive vagal outflow. Through endocardial ablation of the neuromyocardial interface and GP, cardiac autonomic nerve activity can be rebalanced. With the emerging evidence of ANS being involved in the development of certain diseases, the indication of CNA may expand in the future. 
Clinical Perspective
- Cardioneuroablation is a potential intervention to rebalance the cardiac autonomic nervous system and is a promising treatment for vasovagal syncope.
- Symptomatic patients with cardioinhibitory or mixed response of tilt table testing may be candidates for cardioneuroablation.
- The anatomical approach, high-frequency stimulation or AF-nest mapping by fractionation are the most commonly used methods to identify ablation targets. Extracardiac vagal stimulation seems to be an important tool for a rational acute endpoint.