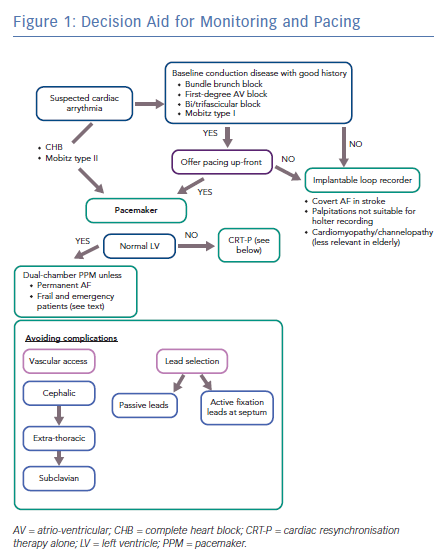
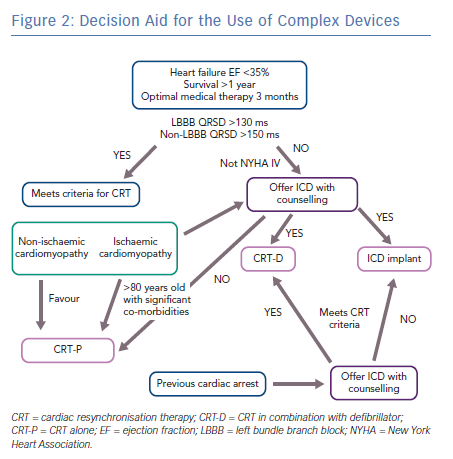
The number of cardiac implantable electronic devices (CIED) has been increasing year-on-year.1 This, coupled with improvements in life expectancy,2 means that more elderly patients will meet the criteria for a CIED. National and international guidelines set out clear criteria and make recommendations for CIED use based on available evidence.3–6 However, the majority of clinical trials include few, if any, elderly patients (>80 years), with supposed benefits in the elderly population extrapolated from data derived from younger patients. The aim of this review is to give an overview of the different types of CIEDs and to discuss our approach on their use in the elderly population (>75 years) going beyond guideline recommendations.
Implantable Loop Recorders
Falls are a common presentation among elderly patients admitted to hospitals.7 These include mechanical falls or those resulting from a transient loss of consciousness. In the majority of cases, a detailed history, physical examination (including lying and standing blood pressure) and simple investigations such as an ECG may help determine the cause.8 The challenge lies with patients in whom a cause is not apparent but an arrhythmia is suspected either clinically or epidemiologically. Given the short duration of Holter monitoring, there is often a low yield in correlating arrhythmia with clinical symptoms. In contrast, implantable cardiac monitors can be useful in determining any correlation between symptoms and rhythms, aiding in the diagnosis of a clinically relevant arrhythmia. They are particularly helpful for patients whose initial investigations have been negative with a normal baseline ECG.9,10
The elderly population are much more likely than younger patients to have brady-arrhythmia as a cause of their syncope. Understanding the background to the fall or loss of consciousness is also critical to the decision-making process, and the following characteristics are typical of an event that may be bradycardia related:
- Sudden loss of consciousness without any preceding symptoms.
- The patient is unconscious before they hit the floor (and may have facial injury as they have not put their hands out to save themselves).
- The patient feels better almost as soon as they regain consciousness.
- The loss of consciousness occurs while sitting or lying (slumping over while have a meal with friends or family is a classic warning that the patient has a cardiac rhythm problem).
In patients with some degree of underlying conduction disease on ECG (bifascicular or trifascicular block, marked first-degree atrio-ventricular [AV] block) then this, combined with a compelling clinical history of syncope, may warrant implantation of a pacemaker without evidence of higher-degree block correlating with symptoms.5 However, the obvious disadvantage of the loop recorder is the necessity of another syncopal episode for its diagnostic utility, minimising its suitability for high-risk patients.
Although loop recorders are often used to investigate infrequent palpitations, in a patient with preserved ejection fraction (EF) and no evidence of inherited arrhythmogenic tendency, palpitations will usually represent a benign symptomatic problem. In such mildly symptomatic patients, implantation of a loop recorder is unlikely to change the management plan. Alternatives such as the hand-held, smartphone-based ECG recording devices can instead be offered to the patient or they can purchase them themselves. These may yield similar results without the need for invasive monitoring. Loop recorders can be useful to screen for asymptomatic AF, particularly in high CHA2DS2-VASc score patients who have had cryptogenic stroke.11
Pacemakers
Over 80% of pacemakers are implanted in the elderly patient (mean age 75±10 years).12 The most common indication is AV block and sinus node disease. All patients with complete heart block and type 2 second-degree AV block should be implanted with a pacemaker regardless of symptoms as this has prognostic significance.5 Beyond this, pacing is generally only carried out if bradycardia is accompanied by symptoms.
Elderly patients are more prone to complications. A meta-analysis by Armaganijan et al. showed that elderly patients undergoing device implantation are at increased risk of complications, in particular pneumothorax and lead dislodgements.13 Pneumothorax conveys significant morbidity in older patients, with prolonged hospital stays and a risk of developing infections. To reduce the risk of pneumothorax, a traditional ‘blind’ subclavian puncture should be avoided wherever possible. Implantation using the cephalic vein, the use of ultrasound-guided punctures or extra-thoracic punctures with fluoroscopic guidance have been shown to reduce the risk of pneumothorax and should be used when possible.14,15 The higher incidence of lead dislodgement in the elderly is often related to an increase in venous tortuosity as well as reduced cardiac mass for lead attachment.13 Additional redundancy should be left on the lead during the implant. Data from the Pacemaker Selection in the Elderly (PASE) trial demonstrated that older age is a risk factor for lead perforation.15 Furthermore, a study by Sterlinski et al. demonstrated that – compared with passive leads – active leads were more likely to result in perforation.16 Therefore, to minimise the risk of perforation we recommend either using a passive fixation lead or an active fixation lead to the interventricular septum avoiding the right ventricular apex.
While there are data demonstrating that dual-chamber may be superior to single-chamber pacemakers in the elderly regarding symptoms related to the pacemaker syndrome,17 there may be circumstances when a single-chamber system is appropriate. In frail, unstable, agitated patients presenting in complete heart block, it would be reasonable to reduce the procedure time and implant a single-lead device to render the patient safe and to avoid a more prolonged procedure that could be distressing.
It is important to offer an individual, tailored approach when pacing is considered in the elderly. These patients are more likely to have multiple co-morbidities that could affect the decision-making process. For example, in a bed-bound patient with an incidental finding of sinus node disease who is having fleeting dizzy spells, any benefit may not outweigh the risk and inconvenience of pacemaker implantation. Therefore, when discussing therapy with any patient – but particularly the elderly where the trauma of intervention may have a bigger impact than the existing symptoms – it is important to provide clear information as to why the procedure may help so that they can weigh this up against the downsides. A decision aid for monitoring and pacing options is shown in Figure 1.
ICDs
ICDs are implanted either for secondary prevention in patients who have a survived cardiac arrest or as primary prevention therapy.5,6 The evidence for the use of ICDs in these situations is well established but, as in many clinical trials, the elderly (>75 years) are poorly represented in these studies with a mean age of 63 in published randomised controlled trials (RCTs).18
Healey et al. first highlighted the issue on the overall benefit of ICDs as secondary prevention by pooling data from published RCTs. They found that elderly patients had a higher incidence of non-arrhythmic deaths, minimising the benefit of an ICD. However, the modest sample of 252 elderly patients (aged ≥75 years) in this study may not reflect modern-day practice.19 Other observational studies from the Ontario database highlighted that age alone cannot be the sole predictor of mortality but rather that other co-morbidities like chronic renal failure, heart failure and chronic obstructive pulmonary disease are significant predictors of mortality.20
An analysis of real-world data on the impact on ICD for secondary prevention involving over 12,000 patients over the age of 65 years showed that, while the rates of death increased with age, four in five older patients survived beyond 2 years. However, the study did not demonstrate the mode of death. Interestingly, beyond mortality, older patients have significant morbidity following an ICD implantation with higher rates of admission to special nursing facilities and re-admission to hospitals.21
While offering an ICD as secondary prevention seems like a logical choice and in line with current guidelines, it should be noted that – although it can prolong life – ICD implantation carries certain risk of increased morbidity. In the elderly population, some patients would value quality of life rather than longevity and therefore an open and honest discussion needs to take place prior to embarking on an implant. It is important to recognise that the ICD may simply change the mode of death from a sudden one to a longer, protracted and ultimately more distressing one, with no impact upon quality of life in the intervening period. This is likely in conflict with the end-of-life expectations of most – if not all – patients.
Primary prevention ICDs are indicated in patients with heart failure with a left ventricular EF <35% except those in New York Heart Association (NYHA) class IV and who have been on optimal medical therapy for a minimum of 3 months and expected to live more than a year.5,6 Mode of death in patients with heart failure can either be driven by a life-threatening arrhythmia or pump failure. Data from the ALTITUDE registry showed that the frequency of ICD therapy in the older age group is lower and other observational studies show that the mode of death in the elderly is more likely to be pump failure.22
Elderly patients are also more likely to have multiple co-morbidities that could impact on 1-year survival. Ferretto et al. studied patients aged >75 years who had an ICD implanted for primary prevention. The authors concluded that age alone was not a predictor of 1-year mortality but rather EF <25% and moderate to severe renal failure predicted a high 1-year non-arrhythmia death of up to 45.5%.23
The use of ICDs in heart failure patients changes the mode of death from an arrhythmia cause to one of progressive pump failure. Furthermore, although older patients have been found to be less likely to have ICD shocks, both appropriate and inappropriate shocks are likely to have a significant impact on physical and mental wellbeing.22 A clear plan for disabling anti-tachycardia therapy when approaching end-of-life care should be discussed with all patients having an ICD implanted so that they are able to make their wishes clear, ideally well in advance of any potential incapacity. It is important to ensure that the patient understands the distinction between bradycardia and tachycardia therapies when having this discussion. Generally, if one has not had the potentially uncomfortable, albeit necessary discussion with the elderly patient about how they ‘want to die’ then it is likely that one has not really given them all the information they need to decide whether an ICD is right for them.
Cardiac Resynchronisation Therapy
Cardiac resynchronisation therapy (CRT) either alone (CRT-P) or in combination with a defibrillator (CRT-D) is well established in the treatment of patients with heart failure. Its use in the elderly is increasing, with up to 40% of CRT being implanted in patients over the age of 80 years.24
Although clinical trials do not exclude elderly patients, the major trials that influence our clinical practice have a predominantly younger population making results derived from these trials unrepresentative of the older age group.
Killu et al. conducted a retrospective analysis to determine the outcomes of CRT in patients aged >80 years. They demonstrated that although overall survival was worse when compared to their younger counterparts, CRT resulted in improvement in NYHA class, EF and mitral regurgitation severity.25
Martens et al. investigated the impact of CRT on both morbidity and mortality. Their findings were similar to those of Killu et al., with improvements in NYHA class and EF compared with younger counterparts. They also showed that elderly patients had higher all-cause mortality but this was no different to age-matched controls who had no heart failure. In addition, they demonstrated that elderly patients had a similar rate of heart-failure-related admissions compared to younger patients. The mode of death in octogenarians was mainly non-cardiac. When a death had a cardiac cause it was because of worsening heart failure rather than malignant arrhythmia.24 Aktas et al. analysed data from the multicentre automatic defibrillator trial and showed that elderly patients (>75 years) had a lower risk of ventricular tachy-arrhythmias compared with their younger counterparts (<75 years) providing further evidence to support the notion that malignant arrhythmias are less frequent in the elderly.26
The publication of the Danish Randomized, Controlled, Multicenter Study to Assess the Efficacy of Implantable Cardioverter Defibrillator in Patients With Non-ischemic Systolic Heart Failure on Mortality (DANISH) generated intense debate on whether ICD has any added value in the elderly non-ischaemic cardiomyopathy population because in the pre-specified subgroup analysis, ICDs did show benefit in younger but not older (>70 years) patients and sudden death rates in the elderly were much lower than their younger counterparts.27,28 Real-world data from a large, single-centre series29 have suggested that CRT-D does not provide additional survival benefit in patients with non-ischaemic cardiomyopathy. Recent data from two centres in the UK with a median long-term follow up of 4.7 years showed CRT-D only had mortality benefit over CRT-P in patients with ischaemic aetiology.30
Given a lack of robust RCTs, international guidelines do not advise on prescription of CRT-P or CRT-D. Data from the CeRtiTuDe cohort showed that CRT-P patients selected in routine clinical practice did not benefit from adding a defibrillator. CRT-P patients in this cohort tended to be older and mainly had a non-ischaemic aetiology.31 In addition, while there are emerging data suggesting that addition of an ICD in elderly patients (>75 years) undergoing CRT implant does not impact on survival,32 it is worth remembering CRT-P alone is an excellent treatment option, as demonstrated in the CArdiac REsynchronisation in Heart Failure (CARE-HF) study.33 The final decision on CRT-D versus CRT-P should not only be guided by heart failure aetiology but, more importantly, our patients’ expectations and desires. A decision aid guiding the use of complex devices is shown in Figure 2.
Remote Monitoring
Remote monitoring has been shown to be easy to use and well accepted in the elderly population. There are two areas where this could have a significant impact in this population group. The first is in AF detection as elderly patients tend to have a high CHA2DS2-VASc score and remote monitoring could prevent delay in appropriate anticoagulation. The second area is in heart failure monitoring to allow for early intervention and avoid hospitalisation. Hospital admission in elderly patients can be prolonged, often leaving them deconditioned even after discharge from hospital.34
Conclusion
To quote the French author Jules Renard, “it is not how old you are, but how you are old”. It is not age itself that affects the decision process but the co-morbidities that come with age that are important. Thunes et al. demonstrated that patients who underwent CRT-D implant who had more co-morbidities (high Charlson co-morbidity index) had a poorer survival.35 Risk stratification using validated scores can help guide the consultation by providing patients with objective data that could impact on their ultimate decision.36
Decision making for device implantation in elderly people should not be driven by guidelines alone. These patients may have complex co-morbidities and personal wishes that cannot be accommodated by guidelines. It is important to have a clear, open discussion with patients about the reasons for device therapy and to ensure this meets their expectations and wishes. This sometimes can be challenging in the presence of other family members where their wishes and views may not be aligned with the patient’s. Therefore there may be times when one has to ensure that the patient has a genuine opportunity to individually assess their treatment options, albeit keeping in mind that having the family engaged and involved is crucial.
Clinical Perspective
- Prolonged cardiac monitoring is useful in making a diagnosis but often a pragmatic approach is recommended in the elderly.
- Peri-procedural complications can have a drastic impact on elderly patients and our approach should be adapted to reduce such risk.
- ICDs save lives but can impact on long-term morbidity.
- CRT confers functional and mortality benefits in the elderly and should not be withheld.
- A holistic, patient-centred approach is essential in care delivery.