Despite multiple advances in treatment strategies, the incidence and prevalence of AF continues to increase worldwide, affecting up to 4% of the population.1–4 AF is associated with significant morbidity and mortality, leading to a notable burden on healthcare systems represented by more hospitalisations, an increased incidence of stroke and loss of productivity. The estimated annual healthcare cost of AF treatment has risen to nearly US$26 billion in the US alone.5–9 Stroke and systemic embolism account for a significant proportion of the increase in morbidity, mortality and healthcare-related costs, as the risk of stroke is increased about fivefold compared to the general population.10 This increased risk of stroke is even higher in elderly patients with other comorbidities, such as hypertension, diabetes, heart failure, chronic kidney disease and coronary/vascular disease. Thus, current treatment guidelines focus on the use of oral anticoagulant (OAC) treatment for stroke prevention in patients with AF.11
Unfortunately, patients who have a higher risk of stroke are also at a higher risk of bleeding, even with the use of newer OACs.12,13 When patients are deemed at high risk of future bleeding events, which precludes the safe use of OAC therapy, left atrial appendage (LAA) occlusion (LAAO) has an established role in stroke prevention.14 LAAO procedures require significant expertise in cardiac anatomy, catheter manipulation within the left atrium (LA) and intraprocedural image guidance to obtain adequate closure (allowing adequate landing-zone evaluation and monitoring device deployment) and reduce procedure-related complications (by facilitating transseptal puncture [TSP] and ruling out thrombus).15 Transoesophageal echocardiography (TOE) has been the most often used imaging strategy to guide LAAO procedures as the pivotal trials that led to the approval of LAAO used this imaging modality.16
Intracardiac echocardiography (ICE) uses 8–12 Fr catheters capable of obtaining 2D or even real-time 3D echo images from within the vascular system. ICE imaging has been widely used in electrophysiology procedures (particularly pulmonary vein isolation, in which the use of ICE is associated with a reduction in procedure-related complications and in-hospital mortality) as well as closure of interatrial septal defects, in which ICE guidance has been demonstrated to reduce intraprocedural and fluoroscopy times and length of hospital stay.17–19 Although there has been a slight increase in the use of ICE to guide LAAO procedures as an alternative to TOE guidance over the past years, ICE guidance only amounts for 2.2% of all LAAO procedures in the US.20 In this review, we summarise the evidence supporting ICE use during LAAO procedures and provide a description of the procedural technique.
Characteristics of Intracardiac Echocardiography Catheters
Most ICE catheters have a 64 phased array design, providing a wedge-shaped image that most operators may feel comfortable with as it is similar to those obtained with TOE probes. The transducer is mounted in 8–12.5 Fr guide catheters, which have a steering knob that allows the catheter tip to flex in four directions (anterior, posterior, left and right). Image quality is excellent and all catheters are capable of providing 2D as well as Doppler imaging, which is useful for demonstrating peri-device leaks. The two most frequently available are the ViewFlex Xtra (Abbott Medical), a 9 Fr catheter, and the Acuson AcuNav (Biosense Webster), available in 8 Fr or 10 Fr diameters. More recently, ICE catheters capable of providing real-time 3D imaging have been introduced, facilitating the evaluation of the LAA ostium.21 These include the VeriSight Pro (Philips), the NuVision (Biosense Webster) and the AcuNav Volume (Siemens Healthineers). By providing images in the long and short axis simultaneously, 3D catheters are expected to reduce the amount of catheter manipulation to obtain proper imaging of cardiac structures and localisation of the catheters. However, the cost of 3D ICE catheters is significantly higher than 2D catheters.
Benefits and Shortcomings of Using ICE Guidance During LAAO Procedures
The main benefit of ICE-guided LAAO is that it can be performed with light sedation as opposed to using general anaesthesia/deep sedation which is required for TOE-guided LAAO. This avoids the risks of complications related to anaesthesia and endotracheal intubation, while at the same time reducing in-room (defined as the time between femoral access and procedure termination) and recovery time.22 Additionally, the personnel required in the lab is reduced because there is no need for an anaesthetist and an imaging cardiologist, reducing the number of people exposed to ionising radiation. This is particularly worrying for the imaging cardiologist, who stands beside the X-ray source and must frequently expose their hands in the X-ray field. Importantly, since the TSP can be performed under ICE-guidance alone, a reduction in fluoroscopy time is feasible in operators with previous experience on fluoroless TSP.23 By reducing the number of medical specialties involved in the procedure, ICE guidance may facilitate procedural scheduling, thus improving procedural workflow.
TOE is also associated with significant risks. Up to 86% of patients undergoing structural interventions can have TOE-associated lesions detected on oesophagogastroduodenoscopy, with 40% of patients having complex lesions, including haematomas or lacerations.24 Most of these lesions are not detected during routine clinical evaluation and may only manifest as discomfort, as the incidence of clinically detected TOE-related major complications in patients undergoing high-risk structural procedures (including LAAO) is only 6.1%.25 Finally, TOE cannot be performed in up to 2% of patients due to anatomical difficulties, lack of patient cooperation or operator experience, or the presence of high-risk conditions (including previous oesophageal lesions or altered respiratory status).26
Cost is frequently considered a significant limitation to the use of ICE imaging, particularly when only the upfront cost of the ICE catheter versus the cost of a TOE is equated. Using an administrative database to analyse claims, ICE has been associated with an excess cost of hospitalisation of US$1,769.20 However, when considering the other benefits of using ICE guidance, such as a reduction in hospital stays and in-room times, ICE has frequently been demonstrated to result in similar overall costs to TOE guidance in structural interventions.19 When comparing only hospital charges, ICE-guided closure appears to be more expensive than TOE-guided closure; however, when professional fees are included, there is no significant difference between both imaging modalities.27
Another study found that the overall cost of ICE-guided closure was significantly lower than TOE-guided closure, driven by significantly lower staffing fees.22 Importantly, studies that have demonstrated similar costs between ICE and TOE guidance for LAAO have not considered the lost profits associated with having a TOE specialist in the lab who could be in the echo lab performing other echocardiograms, or the added cost of longer in-room times. Thus, cost does not appear to be a real limitation to ICE-guided LAAO when all other costs are considered. With the introduction and possible widespread use of real-time 3D echo catheters, the impact of the ICE catheter cost on overall procedural costs is yet to be determined.
ICE-guided LAAO places an additional responsibility on the operator, who not only has to be responsible for the procedure but also for obtaining adequate images, which is a significant disadvantage when compared to TOE-guided closure. This added responsibility may be overwhelming for less experienced operators, and may, at least in part, explain the higher risk of pericardial effusion observed at the start of the learning curve.28 Concerns about other potential shortcomings of ICE, including the ability of ICE imaging to rule out thrombus, appear to be unjustified as ICE imaging appears to be similar to TOE imaging for thrombus detection.29
Starting an ICE-Guided LAAO Programme
Given the added responsibility of obtaining adequate images during the procedure, it is recommended that operators interested in performing ICE-guided LAAO have previous experience with ICE catheter manipulation, ICE image interpretation and LAAO. The use of preprocedural imaging (particularly CT imaging) can facilitate appropriate device selection and is recommended by current guidelines as a part of preprocedural planning.14 Most electrophysiologists have experience with ICE manipulation and imaging, thus facilitating its adoption. For operators without significant experience in ICE imaging, it is recommended to first become familiarised with the basic principles of ICE imaging, including visualisation of the LAA from the right cavities. During left-sided ablation procedures, such as pulmonary vein isolation, the ICE catheter can be inserted within the LA using the same transeptal access. This will allow the operator to practise manipulating the ICE catheter within the LA, while at the same time providing valuable information regarding catheter contact during ablation. For operators who do not routinely perform ICE-guided ablation procedures, it is recommended to perform the first cases of ICE-guided LAAO using both TOE guidance and ICE guidance, to provide back-up imaging guidance. Moreover, guidance by another specialist with experience in ICE-guided LAAO is highly recommended during the initial cases.
Evidence Supporting ICE-guided LAAO
ICE guidance was initially reported as an adjunct to intraprocedural TOE guidance.30,31 The first use of ICE as a standalone imaging modality came in 2010, and several observational studies comparing ICE with TOE guidance have been published since (Table 1).32 In all of these studies, ICE-guided LAAO was performed by operators with previous experience in LAAO which may affect results.
Procedural Efficacy and Safety
Studies have reported a similar procedural efficacy (defined as the ability to deploy the selected device) and safety (defined as freedom from procedure-related complications) in patients undergoing ICE-guided versus TOE-guided LAAO. The overall procedural efficacy for both imaging modalities is very high (98.3% versus 97.8% for ICE-guided versus TOE-guided closure, respectively), with a very low risk of procedure-related major adverse events and no significant differences between groups (3.5% versus 6.1% in the ICE versus TOE groups, respectively).33
A study using an administrative claims database found a significant reduction in gastrointestinal or bleeding complications in patients undergoing ICE-guided LAAO compared to TOE-guided LAAO (OR 0.59; 95% CI [0.37–0.94]).20 Operator experience appears to play a significant role in procedural safety, with a recent database claim study reporting a higher risk of pericardial effusion in patients undergoing ICE-guided LAAO (1% versus 0.5%, p=0.02). In this study, 82% of operators performing ICE-guided LAAO performed fewer than ten procedures during the study period, with a higher number of procedures associated with a lower risk of procedure-related adverse events.28 A recent meta-analysis of observational studies demonstrated no difference in the risk of pericardial effusion/tamponade (1.2% versus 1.4%) in the ICE and TOE group, respectively; (OR 1.07, 95% CI [0.52–2.19], p=0.85; I2=0%); however, most operators had significant experience with ICE-guided LAAO.
Recently, a single-arm prospective multicentre study with central adjudication of echocardiographic images (ICE-LAA) demonstrated a 100% technical success of ICE-guided LAAO, defined as successful deployment and release, no conversion to TOE and effective closure of LAA at implantation.34 When compared with the results of the PINNACLE-FLX trial – in which TOE was the main imaging modality – the rate of procedure-related major adverse events was similar. Additionally, the study validated the use of the PASS (position, anchoring, sizing and sealing) release criteria as determined with ICE imaging.
Procedural, Fluoroscopy and In-room Time
Determining the impact of ICE versus TOE guidance on procedural and fluoroscopy time during LAAO is difficult as ICE guidance is a relatively new technique in which most operators have little previous experience. Conversely, studies evaluating ICE guidance during LAAO have been performed with operators with significant prior experience with LAAO, which may (at least partially) offset the increased procedural and fluoroscopy times associated with the acquisition of a new set of skills to manipulate the ICE catheter within the LA. To date, there has been no difference in procedural or fluoroscopy time between both imaging modalities. However, ICE guidance appears to be associated with a significant reduction in in-room time compared to TOE guidance (mean-weighted 28.6-min reduction in in-room time).33
Peri-device Leaks and Residual Interatrial Septum Defects
Although some concerns may exist on the ability of ICE to detect peri-device leaks intraprocedurally, the rate of peri-device leaks does not differ from TOE-guided LAAO on follow-up imaging.33 In the ICE-LAA study, 23.8% of patients had 0–5 mm leaks, with no patients demonstrating >5 mm leaks; as such, effective closure – defined as the absence of peri-device leaks on follow-up imaging – was achieved in 76.2% of patients.34 The percentage of patients without leaks was similar to that observed in previous LAAO registries, and although small leaks (<5 mm) were previously considered to have a benign course, they are also associated with a higher risk of stroke (adjusted HR 1.152, 95% CI [1.025–1.294]).35 In the PINNACLE FLX study the prevalence of small leaks at 45-day imaging was 17.2% and 10.5% at 1 year.36 Although the reason for the differences in peri-device leaks between studies is unknown, efforts to reduce their prevalence by achieving appropriate alignment of the device with the LAA, as well as ensuring adequate device sizing, should be undertaken.
ICE-guided LAAO appears to be related to a higher risk of residual IAS defects on follow-up imaging. However, on follow-up imaging these defects can close in up to 70% of patients, without differences between imaging techniques and no apparent haemodynamic impact.37
Technique to Perform ICE-guided LAAO
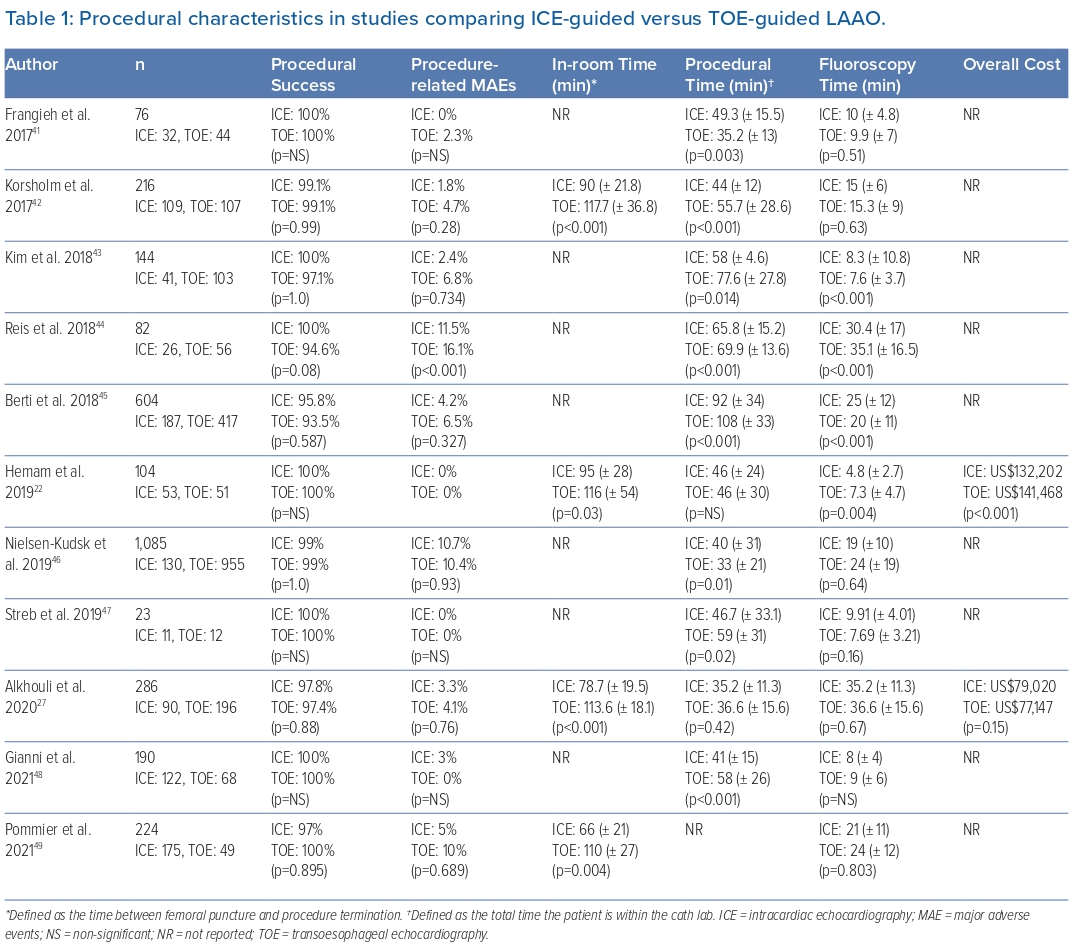
Under light sedation, right-sided femoral access for the ICE catheter (placed in a medial location) and the TSP is obtained. Obtaining right femoral access for the ICE catheter provides a straighter route to the heart, thus facilitating the procedure. Advancement of the catheter from the femoral vein to the right atrium (RA) can be performed either with fluoroscopic guidance or under ICE imaging alone, depending on operator experience. The use of long femoral sheaths can facilitate delivery of the ICE catheter near the RA and could be useful for operators with less experience. The ICE catheter is advanced into the middle of the RA facing the tricuspid valve – the right atrial home view. From this position, the catheter can be advanced into the right ventricle (RV) using anterior tilt. Once in the RV, releasing the anterior tilt with careful advancement into the RV outflow tract while providing clockwise rotation will allow visualisation of the LAA. Insertion into the RV can also be useful to rule out pericardial effusion as a short axis of the left ventricle can be obtained (Figure 1). Alternatively, the catheter can be advanced into the pulmonary artery. These two echo views can be used for initial LAA morphology and rule out thrombus. However, to adequately guide the LAAO procedure the ICE catheter must be inserted into the LA through a TSP as the views obtained from the right cardiac chambers are insufficient.
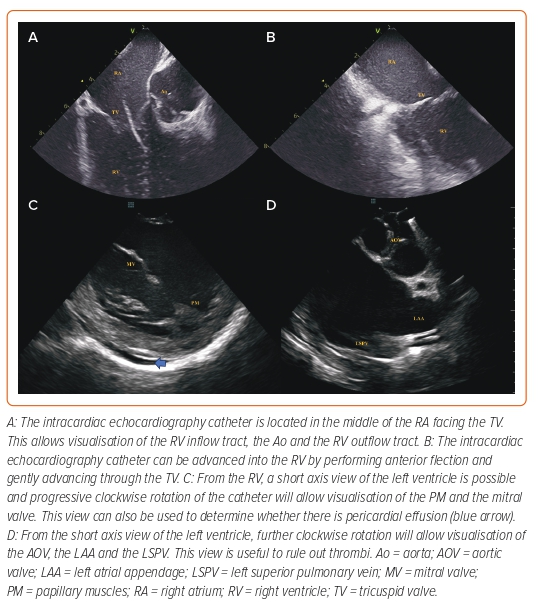
From the home view position, clockwise rotation will reveal the interatrial septum (IAS) (Figure 2). Counterclockwise rotation from this position will bring the aortic valve into view, while clockwise rotation will bring the posterior LA wall into view. Thus, the ICE catheter can be easily used to determine if the puncture site is too anterior as the aorta appears in the visual field, or too posterior as the posterior wall appears in the visual field. Although lateral deflection can be applied to the catheter to delineate the anterior and posterior margins of the IAS, it is frequently difficult to precisely locate the transseptal needle and thus we prefer using the ‘long axis’ view (similar to the bicaval view in TOE) and use clockwise/counterclockwise rotation to determine the exact position of the needle. From the IAS view, posterior tilt (and occasionally rightward flexion) of the catheter will permit visualisation of the superior vena cava. The TSP sheath can be followed as it descends into the IAS and the sheath position should be adjusted to achieve a desirable location. Although TSP for LAAO is frequently performed in a posterior location, a more anterior location in which the LAA ostium is visualised in the far-field image is desirable as it allows coaxial deployment of the device within the LAA main body. TSP is then performed under continuous ICE guidance.
The ICE catheter can be inserted into the LA either through a separate TSP or through the same puncture as the delivery sheath. When a separate TSP is used, the ICE catheter is used to guide two independent TSPs: one for the delivery sheath, and the other through which the ICE catheter will be advanced into the LA. However, we believe that the use of two TSPs is time-consuming and prefer the single TSP approach: the ICE catheter is inserted through a standard femoral sheath and used to guide a TSP. A high support guidewire is advanced to the left superior pulmonary vein (when using a straight guidewire) or the LA (when using a spiral high support guidewire or the Versacross [Boston Scientific]). When using straight guidewires, we prefer the use of a Supracore guidewire (Abbott Medical) as the flexible straight tip navigates easily through the pulmonary veins, while having a radio-opaque marker which determines where the region of high support begins, reducing the risk of prolapsing the guidewire into the RA due to insufficient support within the LA. When using a straight guidewire, it is important to avoid excesive advancement into the pulmonary veins, as this could result in bronchial bleeding.38
After the high support sheath is in place, the transseptal sheath is used to dilate the TSP (Supplementary Video 1) by crossing the IAS several times. Once dilated, the sheath is withdrawn to the inferior vena cava, leaving the guidewire within the pulmonary vein. The ICE catheter is then advanced into the LA through the same TSP using the guidewire as a reference to cross the fossa ovalis (Supplementary Video 2) and once in the LA, the delivery sheath is advanced over the wire. Crossing the IAS may be one of the most difficult parts to master during ICE-guided LAA and should be practised using both echo and fluoroscopy guidance.
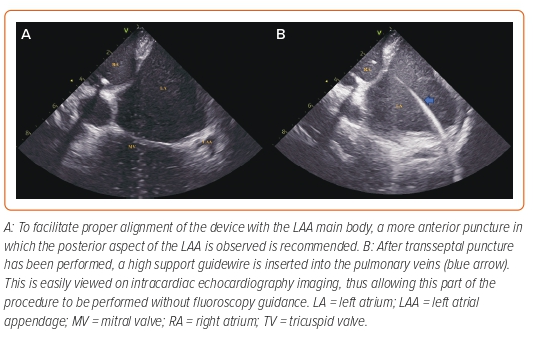
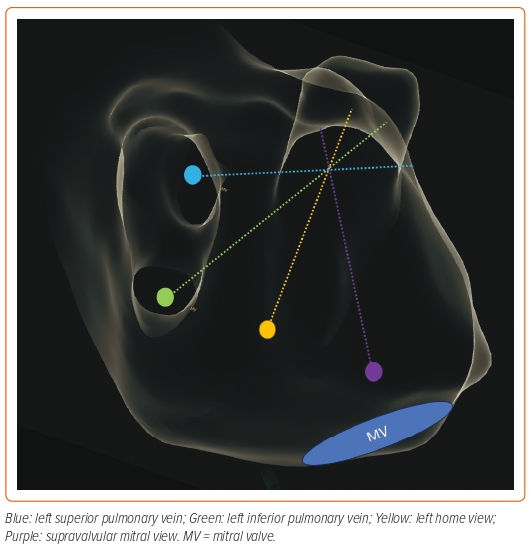
There are three main ICE positions within the LA that should be used during LAAO (Figure 3). Although we provide a comparison with the corresponding TOE image for each of the ICE positions, we recommend that operators consider ICE as a different technique as it may help in the learning process, instead of looking for similarities and trying to replicate the images obtained with TOE to which they are accustomed.
LA Home View
After crossing the IAS, the ICE catheter is placed in the mid-LA facing towards the LAA ostium. Slight posterior deflection of the catheter allows visualisation of the LAA. This is a stable position and can be used during device delivery to monitor the position and make appropriate adjustments. The LA home view provides a similar visualisation to the 45° TOE view.
Left Superior Pulmonary Vein View
From the LA home view position, the catheter is gently advanced into the left superior pulmonary vein and rotated to bring the LAA into view. This view facilitates evaluation of the LAA depth and is similar to the 0° TOE view.
Mitral View
From the home view, the catheter is rotated until the right-sided pulmonary veins come into view and posterior tilt is applied. The catheter is then gently advanced so that it rests near the lateral mitral annulus. Importantly, the catheter should not be advanced into the left ventricle and care should be exercised when advancing the catheter to avoid perforation of the LA wall. From this position, leftward tilt of the catheter can facilitate visualisation of the LAA. This view is similar to the 135° TOE view.
Other Considerations
Importantly, given the freedom of movement of the ICE catheter within the LA, the ICE catheter can be placed in other locations, including near the roof of the LA or the left inferior pulmonary vein to achieve additional views of the LAA (Figure 4).39
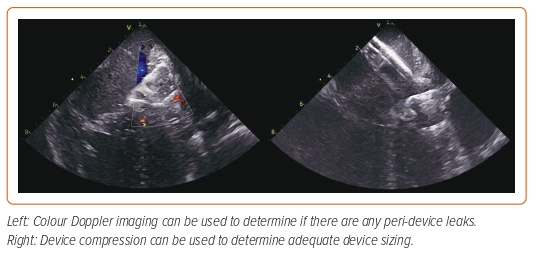
As is the case with TOE, measurements of the landing zone should be performed in at least three views and repeated if necessary. The size of the device should be selected based on the largest measurement obtained and fluoroscopy-based measurements should also be obtained. Device deployment is performed using standard techniques and both fluoroscopic and ICE guidance. After the device has been released, 2D imaging can be used to determine device location, compression and stability, while Doppler imaging can be used to determine the presence of peri-device leaks (Figure 5 and Supplementary Video 3).
Future Directions
The ongoing development of ICE catheters capable of providing real-time 3D imaging is expected to have a profound impact on structural interventions, including LAAO. By providing 3D imaging, the use of this technology may help obtain adequate landing zone measurements and facilitate device selection. Moreover, the need to rely on preprocedural imaging and fluoroscopy views may be reduced, particularly for experienced operators. Indeed, these newer probes could potentially result in LAAO with zero fluoroscopy.40 Although the cost of these catheters exceeds the cost of conventional 2D ICE catheters, increased availability of such catheters may ultimately result in significant cost reductions.
Whether ICE guidance becomes the primary imaging modality during LAAO is yet to be determined. Previous experience with ICE-guided closure of IAS defects demonstrated an initial increase in ICE use between 2003 and 2010 which later plateaued and is currently used in only 50% of these procedures.18 Similarly, the use of ICE guidance during LAAO has not increased significantly over the last few years. Although the recent approval of ICE guidance during LAAO procedures may facilitate the adoption of the technique by some operators, lack of confidence on the technical aspects of ICE-guided LAAO may still play a major role in impeding its adoption.
Conclusion
Compared to TOE guidance, ICE guidance during LAAO procedures is associated with similar procedural safety and efficacy and is increasingly being used. Moreover, ICE guidance avoids general anaesthesia, which eliminates its inherent risks and may improve lab efficiency. Although ICE-guided LAAO is mainly performed by highly experienced operators, the use of this imaging modality may increase as operators become more confident with the use of ICE imaging during structural interventions. Randomised controlled trials comparing both imaging techniques are warranted.

Clinical Perspective
- Left atrial appendage occlusion (LAAO) is a suitable alternative to oral anticoagulation for stroke prevention in patients with AF who are deemed to be at high risk of bleeding complications.
- Most LAAO is performed under transoesophageal echocardiography (TOE) guidance. However, TOE is associated with significant risks and requires the use of general anaesthesia/deep sedation. To overcome these limitations, intracardiac echocardiography (ICE)-guided closure has been proposed as an alternative.
- ICE-guided LAAO has similar procedural success and safety as TOE-guided LAAO with shorter in-room times (defined as the time between femoral access and procedure termination) and similar costs. Although only a minority of LAAO procedures are currently performed under ICE guidance, its use is expected to increase as operators gain experience using the technique.