Accessory pathways (APs) are fibrous connections of myocardial tissue found between the atria and ventricles that allow direct electrical communication between the two chambers. These pathways may permit atrioventricular reciprocating tachycardia (AVRT) or, in some cases, rapidly conducted AF, which can be life threatening due to the potential degeneration to VF.1–3 Most commonly, APs are found along the mitral or tricuspid valve. Approximately 60% of common APs are found along the left lateral free wall along the mitral valve, 25% are found along the septal aspect of the mitral or tricuspid annulus and 15% insert along the right free wall.1,4–6
Catheter ablation is the treatment of choice for patients with symptomatic APs causing recurrent AVRT or in situations where APs conduct rapidly, posing a risk of sudden cardiac death.1,3 Jackman et al. reported the use of radiofrequency (RF) energy and point-by-point mapping of APs with excellent success rates and safety profile.4 This technique remains the mainstay of practice throughout the world.4
Presently AP mapping relies on point-by-point assessment of local electrograms (EGMs) looking closely for pathway EGMs or early atrial or ventricular EGMs during ventricular or atrial pacing or in tachycardia. Most commonly, procedures are undertaken with direct fluoroscopic catheter visualisation or, increasingly, 3D mapping systems to minimise exposure to fluoroscopy.7
Despite these advances, procedures can be time consuming in order to correctly identify the regions to ablate and prone to insufficient mapping. Although the acute ablation success rates have been reported to be more than 95%, late recurrences of conduction across APs has been reported to be between 3% and 9%.8 This may be due to various reasons, including poor catheter tissue contact and mapping errors.9 In addition, because most APs are only up to 3 mm thick, identifying the precise location before ablation is paramount.10
High-density mapping using the open window mapping (OWM) technique is a novel and alternative approach to mapping and ablating APs.9 This review examines the conventional mapping technique and the newer OWM technique with examples of APs ablated using the OWM method, specifically addressing practical mapping techniques and considerations.
Conventional Mapping with and without a 3D Navigational System
Conventional mapping is a commonly used technique to map APs. This involves recording bipolar EGMs on either a single pair of electrodes or across multielectrode pairs simultaneously. Conventional mapping can be performed with and without the use of a 3D navigational system. 3D navigational systems allow operators, who use a conventional mapping technique, to catalogue their data acquisition with precise X,Y,Z coordinates. The addition of 3D navigation-enabled catheters, including multipolar catheters, allows for higher point density and higher resolution EGM sampling. Table 1 outlines the advantages and disadvantages of using the conventional mapping technique with and without 3D navigation systems.
Fundamentals of Mapping Accessory Pathways without 3D Navigational Systems
Mapping may be performed to accurately localise the insertion point of APs either by identifying the earliest retrograde atrial activation/earliest anterograde ventricular activation or by directly recording AP potentials.5,11
In APs with retrograde conduction, the site of the earliest atrial activation can be mapped during tachycardia (if well tolerated) or during ventricular pacing.12 In anterograde APs, the ventricular insertion site can be mapped by first identifying the onset of the delta wave on the ECG. The site of earliest ventricular activation is then mapped and compared with the onset of the delta wave.12
Alternatively, an AP potential may be identified. In anterograde conducting pathways, this is seen preceding the delta wave. In retrograde pathways, potentials are seen between the ventricular and atrial potential. However, given the low amplitude of the AP potential, these can be difficult to identify.12
Accessory Pathway Mapping with a 3D Navigational System
The use of a sensor-based mapping catheter allows for 3D reconstruction of anatomy in real time. As anatomy is reconstructed, electroanatomical (EA) data are acquired with location data based on a 3D Cartesian plane. Critical to mapping APs is the fiducial location, otherwise known as the reference or ‘0’ point. The ‘0’ point serves as a reference to EA data acquired thereafter, with local activation timing (LAT) compared relative to the ‘0’ point. 3D navigational systems have automated annotation algorithms. Each algorithm is proprietary; however, what is consistent across the proprietary algorithms is the speed of acquisition and accuracy of annotation. Operators can now reconstruct 3D anatomy while acquiring a high-density LAT map of AP conduction.
Window of Interest
The window of interest (WOI) is an arbitrarily defined range set around the reference. As locations are sampled by the mapping catheter, the LAT annotation is bound to the WOI. In the setting of mapping APs, fused signals can prove challenging for automated annotation algorithms, resulting in the need for manual user correction. In clinical practice, EGM artefacts, otherwise known as ‘noise’, are a common source for incorrect automated annotation. Optimising the WOI to enclose the insertion site target is a commonly used technique that minimises automated EGM misannotation. This can be cumbersome and may require multiple adjustments prior to the optimal WOI setting. Furthermore, experience is required for this technique to be implemented successfully. However, with the advent of high-density mapping catheters and proprietary software algorithms, the need to adjust the WOI to improve automated annotation is less of an operator concern.
Open Window Mapping
Maury et al. reported the use of high-density 3D electroanatomic mapping in identifying an epicardial posterolateral coronary sinus (CS) AP.13 The patient had previously undergone RF ablation using traditional point-by-point mapping; however, pre-excitation reoccurred some weeks later. Using the Rhythmia system (Boston Scientific), electroanatomical maps of the left atrium, CS and left ventricle were created.13 During distal CS pacing, activation mapping was performed and the timings of activation were manually reannotated against the same reference (pacing artefact) in each chamber to allow visualisation of the complete AP on a single map.13
Subsequently, Schriker et al. reported the use of the OWM technique using the EnSite Precision (Abbott Laboratories) electroanatomical system in 23 patients with right- and left-sided APs.9 OWM was 100% effective at identifying the successful site of ablation.9 There was elimination of APs after a mean RF energy time of 18.5 s, with an average mapping time of only 7.3 min.9 Subsequently, the OWM technique has been further reported in case reports.14–16
OWM has significant advantages over conventional mapping techniques because there is no need to identify atrial, ventricular or pathway EGMs, which can be difficult to see in some cases, particularly with widely spaced bipolar mapping ablation catheters, and can depend on operator experience. Rather, in OWM, both atrial and ventricular EGM signals are included in the WOI and there is automatic algorithm-driven detection of local signals demonstrating the maximum change in voltage over time (dV/dt).9
Another advantage of the OWM technique is in the setting of haemodynamically unstable tachycardia. Kantharia and Shah reported the case of a 36-year-old woman with haemodynamically unstable orthodromic AVRT.17 Conventional point-by-point mapping was unsuccessful due to the instability during tachycardia and induction of supraventricular tachycardia during ventricular pacing. Using the OCTARAY Mapping Catheter (Biosense Webster), the OWM technique was used, allowing the re-entrant circuit to be recorded within only a few beats of tachycardia.17
The disadvantages of the OWM technique include the increased cost associated with using the 3D electroanatomical mapping system, which may be substantial. To date, the OWM technique has also not been validated in patients with multiple pathways. Slanted pathways may also be difficult to map depending on the mapping catheter used. Using the HD Grid Catheter (Abbott, Inc.) in these cases may allow better visualisation of the pathway given the flat design and its ability to collect orthogonal EGMs.9
OWM of APs can be implemented using any 3D electroanatomical mapping system. Here we describe general OWM considerations and the methodology required using the CARTO (Biosense Webster), EnSite Precision (Abbott laboratories) and Rhythmia (Boston Scientific) systems.
General Open Window Mapping Considerations
It is ideal to map the APs while in tachycardia, if tolerated, to avoid fusion of atrioventricular node conduction and AP conduction during mapping. However, if this is impractical, OWM can be performed effectively with atrial or ventricular pacing.9
A stable timing reference is paramount, whether it is an intracardiac ECG (such as from the coronary sinus) or a surface ECG. It is important to exclude the pacing spike from the roving activation interval and collect points only when the catheter is in a stable location. Minimising the interpolation of geometry and timing points, as well as reducing the projection of internal timing points to the geometry surface, can improve accuracy.
It is important to attempt the annotation of local rather than far-field signals. Both –dV/dt and absolute dV/dt are good options as roving detection algorithms, but the absolute peak could also be used. Automatic annotation of high-frequency activity within low-voltage zones has shown promise in retrospective analyses.18
Respiratory gating for both electroanatomical points and geometry reconstruction is critical and further enhances the visualisation of the pathway orientation across the valve plane. Using a higher geometry resolution will limit interpolation and minimise user editing.
The bipolar noise floor is an important consideration that also minimises annotation of signals with poor contact. A higher noise floor can be used with the OWM technique because, in most cases, signals are being measured in the setting of healthy tissue.
The WOI are the main parameters used to create activation maps. This technique was initially used for identifying the critical isthmus in re-entrant arrhythmias.19,20 In OWM, the WOI is opened up to include atrial and ventricular EGMs, which results in an LAT map. Depending on the 3D mapping system used, there are additional algorithms that can be used to visualise the AP’s conduction on a 3D mapping system. Proprietary algorithms denoting a line of block or conduction breakthrough use LAT points acquired within the WOI. It is therefore important to consider the programmed threshold to denote a line of block or visualisation of AP conduction. Thresholding can be expressed as a percentage difference between two LAT points of the mapped cycle length. If the percentage is programmed to low (e.g. 20%), this could show a line of block; in contrast, if the programmed value is too high, this could lead to poor depiction of precise AP conduction breakthrough. Operators should pay careful attention to the WOI and algorithms that are used to enhance interpretation of 3D LAT maps at the time of OWM.
Specific Mapping System Considerations
Open Window Mapping with the CARTO 3 Mapping System
The CARTO 3 mapping system uses the wavefront annotation algorithm.21 This method assesses both the unipolar and bipolar EGMs of an electrode pair. The algorithm scans left to right of the window assessing the dV/dt of the unipolar EGM. The dV/dt that has the greatest slope on the unipolar EGM must also be on the coinciding bipolar EGM. The local activation time is then annotated to the unipolar EGM. Data acquired can also be automatically reassessed with activation of the map consistency algorithm. Points incongruent with their neighbours will be filtered out.
The continuous mapping settings should use a specific cycle length for the mapped rhythm with a narrow position and LAT stability criteria. Enabling pattern matching and referencing to the body surface ECG for the mapped rhythm will further enhance correct beat detection or reference shift.
When using the OWM technique, electrodes that are small and tightly spaced will further minimise the need for user reannotation. Enabling tissue proximity indication or a narrow internal point filter is recommended.
Enabling the extended early meets late (EEML) algorithm in conjunction with HD COLORING automatically assesses the timing across two adjacent local activation timing points. The timing difference is calculated and then expressed as a percentage of the total mapped cycle length. This feature provides the operator with a locality visual for proximal and distal insertion sites of the AP. It is recommended to start at a low EEML% and then gradually increase until ‘AP bridging’ is visualised.
Open Window Mapping with the Advisor HD Grid Catheter and the EnSite X Mapping System
Omnipolar technology (OT) combines bipolar and unipolar signals from groups of three neighbouring electrodes (cliques) on the HD Grid catheter. This unique and novel form of signal processing provides an analysis of maximum voltage, wavefront direction and speed independent of catheter/wavefront orientation.22 The information is local and beat to beat, obviating the need for post-processing. This has advantages in the mapping of APs, because the signal will tend to be processed along its direction of travel, maximising the voltage. Due to its flat shape, the HD Grid catheter also lies perpendicular relative to the pathway. OT activation direction vectors have been shown to help locate the electrical insertion point in a case of atriofascicular tachycardia.22
Suggested mapping settings during tachycardia when using the EnSite X mapping system include score >80, cycle length ±20 ms, speed limit 10 mm/ms, distance OFF, signal to noise 3–5, force OFF, enhanced noise rejection ON, internal projection 3, model fill 10 and OT confidence 10–30.
The suggested mapping settings during pacing include score: >80, cycle length ±20 ms, speed limit 10 mm/ms, distance OFF, signal to noise 3–5, force OFF and enhanced noise rejection OFF.
Open Window Mapping with the Orion Catheter and the Rhythmia HDx Mapping System
Mori et al. reported the use of the Rhythmia mapping system in identifying APs in 128 patients creating high-density atrioventricular dual chamber maps with subsequent ablations at the earliest activation site on the atrioventricular annulus.23 Similarly, the OWM technique can also be used with the Rhythmia mapping system. The novel LUMIPOINT software module (Boston Scientific) may also assist in identifying the AP locations.24,25 The LUMIPOINT software is incorporated in the Rhythmia mapping system. It detects all activations in every EGM regardless of local activation time and can be used to identify critical isthmus locations.24,26
Case Examples
Case 1: Anterior Septal Accessory Pathway in Close Proximity to the Atrioventricular Node
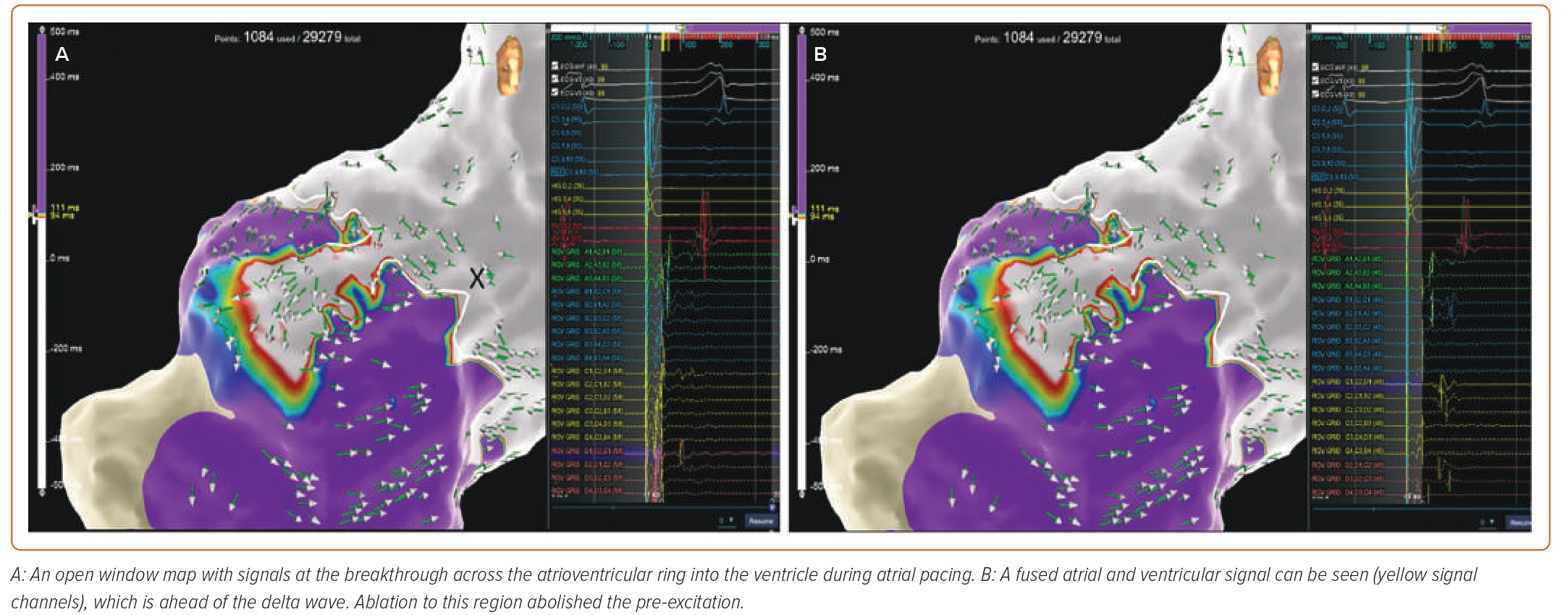
Supplementary Video 1A demonstrates OWM of an anterior septal AP in proximity to the atrioventricular node in a 17-year-old male. Figure 1A shows the OWM map with signals at the breakthrough across the atrioventricular ring into the ventricle during atrial pacing. A fused atrial and ventricular signal can be seen in Figure 1B (yellow signal channels), which is ahead of the delta wave. It can be seen that this region is in a safe anatomical area in relation to the HIS (marked by the ‘X’), and ablation to this region abolished the pre-excitation. Supplementary Video 1B shows loss of pre-excitation during the ablation.
Case 2: Left Posterior Septal Accessory Pathway
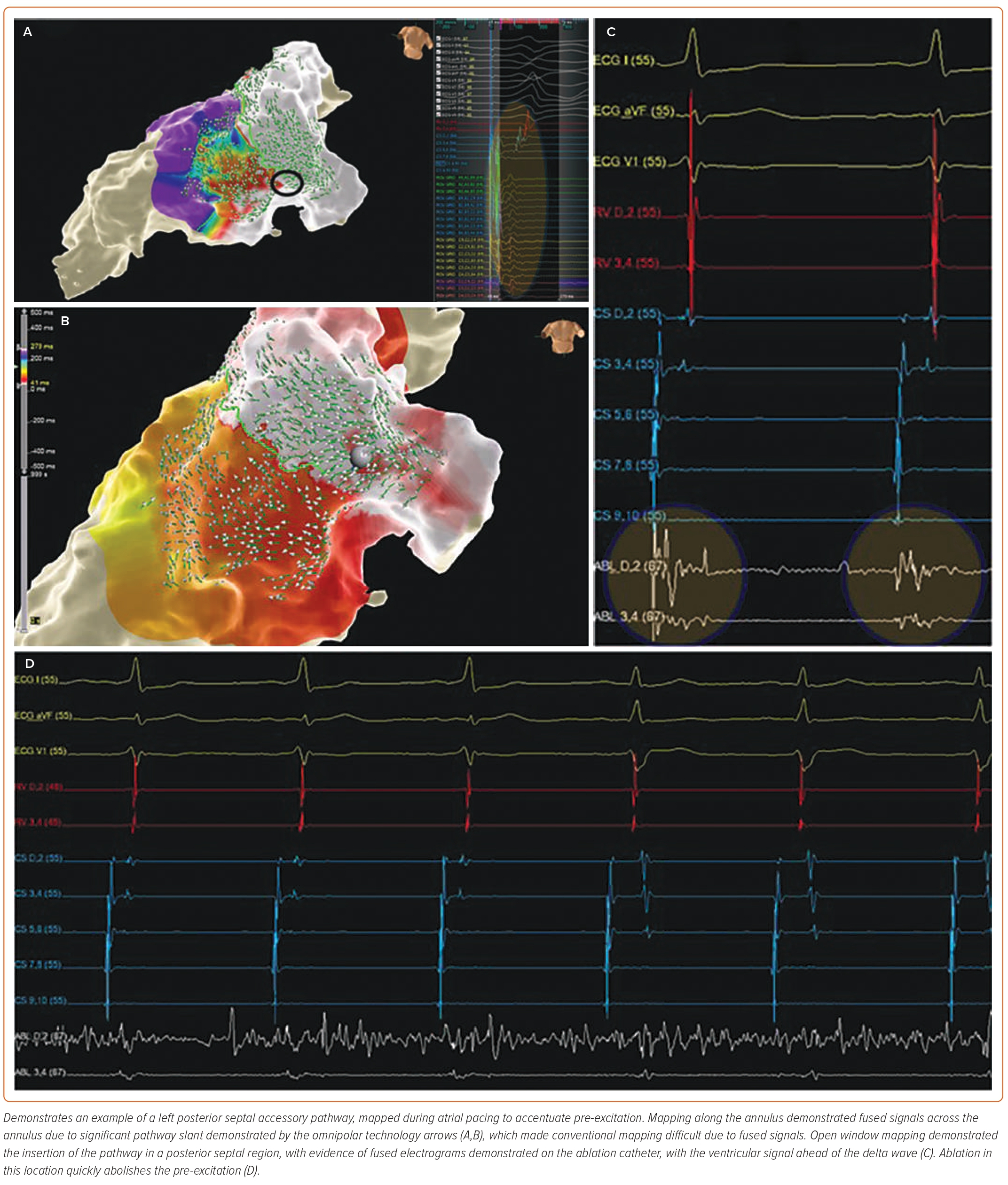
Supplementary Video 2A and Figure 2 demonstrate an example of a left posterior septal AP, mapped during atrial pacing to accentuate pre-excitation. Mapping along the annulus demonstrated fused signals across the annulus due to significant pathway slant demonstrated by the OT arrows (Figure 2A,B), which made conventional mapping difficult due to fused signals. OWM demonstrated the insertion of the pathway in a posterior septal region, with evidence of fused EGMs demonstrated on the ablation catheter, with the ventricular signal ahead of the delta wave (Figure 2C). Ablation in this location quickly abolishes the pre-excitation (Figure 2D). Supplementary Video 2B shows burn termination and loss of pre-excitation during ablation.
Case 3: Left Lateral Concealed Pathway
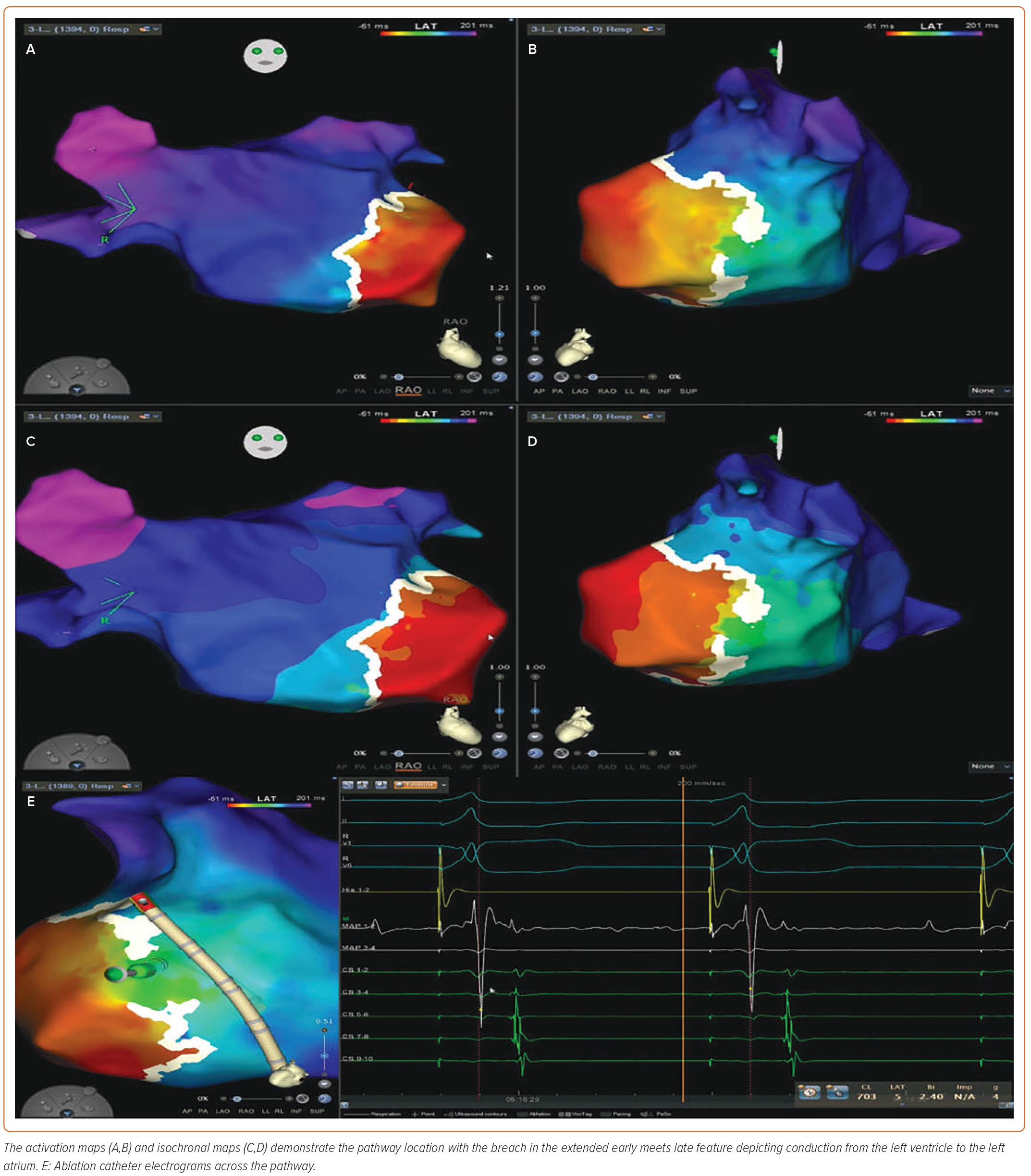
Figure 3 demonstrates an example of a concealed left lateral pathway. The activation maps (Figures 3A and 3B) and isochronal maps (Figures 3C and 3D) demonstrate the pathway location with the breach in the EEML feature depicting conduction from the left ventricle to the left atrium. Figure 3E shows the ablation catheter EGMs across the pathway. Supplementary Video 3A depicts the propagation map using the CARTO 3 mapping system. The DECANAV mapping catheter (Biosense Webster) is positioned in the coronary sinus, demonstrating bracketing of a left-sided AP at the CS 3–4 and CS 5–6 positions. The PentaRay catheter (Biosense Webster) is positioned in the left atrium over the area depicting conduction from the left ventricle to the left atrium via an AP. PentaRay electrodes 1–2 and 3–4 demonstrate fused ventricular and atrial signals. A multicomponent atrial signal is noted at P-1-2. A Quadrapolar catheter (Biosense Webster) is positioned at the base of the right ventricle for pacing during OWM creation. Supplementary Video 3B shows loss of pre-excitation during ablation.
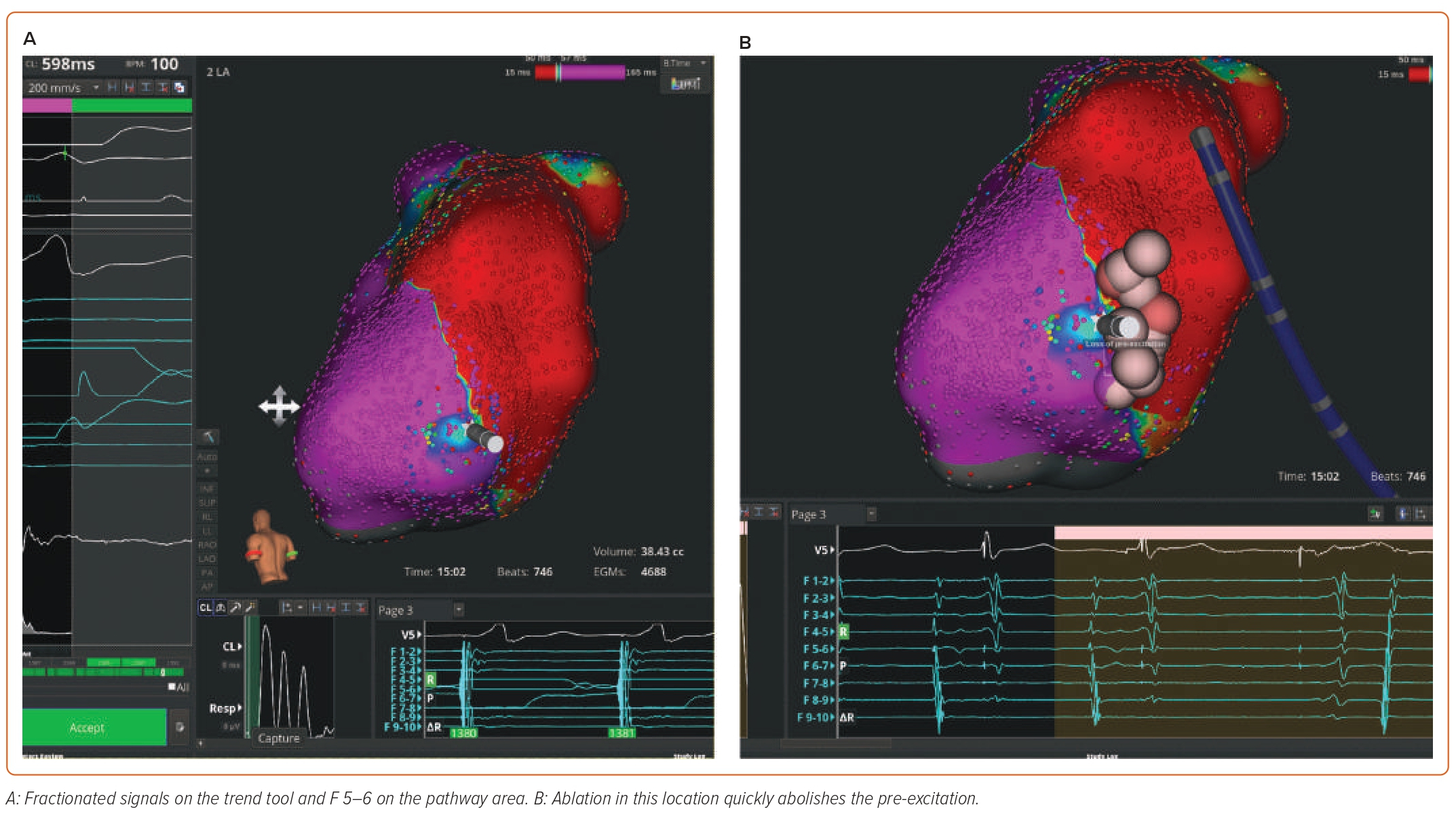
Case 4: Left Lateral Accessory Pathway
Supplementary Video 4 and Figure 4 demonstrate OWM of a manifest left lateral pathway using the Rhythmia mapping system. Figure 4A shows fractionated signals on the trend tool and EGMs F5–6 on the pathway area. Ablation in this location quickly abolishes the pre-excitation (Figure 4B). Supplementary Video 4B shows burn termination and loss of pre-excitation during ablation.
Conclusion
We have highlighted the technical aspects and mapping considerations for OWM, including specific cases demonstrating its utility. OWM represents a novel methodology of visualising APs and, when used with multipolar mapping catheters, enables rapid high-density point collection to quickly identify APs. This has the potential to reduce procedure and fluoroscopy times while quickly identifying critical AP EGMs, potentially reducing ablation times. Given the excellent success rates of point-by-point catheter ablation of APs, it remains to be seen whether OWM will affect outcomes. In addition, the costs of using a multipolar mapping system in healthcare systems need to be borne in mind. 
Clinical Perspective
- Catheter ablation is the treatment of choice for patients with symptomatic accessory pathways.
- Open window mapping represents a novel methodology of visualising accessory pathways.
- Using the open window mapping technique helps to quickly identify the critical electrograms and isthmus.
- The open window mapping technique has the potential to reduce ablation times, procedure times and fluoroscopy times.