Cardiac resynchronisation therapy (CRT) is a well-established treatment strategy in patients with persistent heart failure (HF)-related symptoms (defined as New York Heart Association class II–IV), despite optimal medical therapy, and a left ventricular ejection fraction (LVEF) ≤35% in patients with left bundle branch block (LBBB) or ≤40% in patients with an expected right ventricular (RV) pacing ≥40%.1 Conventional CRT is generally achieved by placing a lead over the left ventricular (LV) epicardium through a cardiac vein and a lead in the RV, thus providing biventricular pacing (BiVp).
Although multiple studies have demonstrated the impact of CRT in reducing HF-related hospitalisations and all-cause mortality, BiVp has significant shortcomings, including acute procedural failure (described in approximately 3–4% of cases using modern tools), phrenic nerve stimulation (in almost 40% of patients) and increasing pacing thresholds or loss of capture.2–8 A considerable number of patients lack adequate venous anatomy for lead implantation, with ideal venous branches frequently in proximity to the phrenic nerve and/or lack of adequate branches in lateral areas, causing the LV lead to be implanted in suboptimal apical or anterior branches. Furthermore, during BiVp myocardial depolarisation and repolarisation is reversed, and occurs from epicardium to endocardium. This results in an artificial depolarisation wave transmitted through the myocardium, leading to a less physiological myocardial activation compared with impulses transmitted via the specialised cardiac conduction system (CCS). Thus, approximately one in three patients fail to respond to CRT.3
Over the past decade, there has been a growing interest in stimulating the CCS. While most studies have included patients without HF, direct stimulation of the CCS could play a pivotal role in patients with LV dyssynchrony that require CRT.9 The first approaches to CCS stimulation were conducted using His bundle pacing (HBP). Although HBP may achieve physiological stimulation of the CCS, the His-SYNC trial showed that 48% of patients failed to effectively activate the distal CCS, thus requiring a crossover to BiVp.10 Furthermore, when HBP does achieve LBBB correction, high pacing thresholds are frequently observed, which results in faster battery depletion.10–12 Additionally, a risk of distal conduction block during follow-up exists, with HBP mandating implantation of backup RV leads.
Left bundle branch area pacing (LBBAP) offers several potential advantages over HBP, including lower pacing thresholds, lack of atrial capture/far field atrial oversensing, and similar degrees of electrical and mechanical synchrony despite longer QRS duration. The physiology underlying the ability to capture the LBBB distal to the site of block has been reviewed in the journal.13 In comparison with BiVP, LBBAP results in lower pacing thresholds, shorter QRS durations and significantly greater improvements in LVEF.14–18 LBBAP seems to be comparatively easier from a technical standpoint than HBP, with a shorter learning curve, attributed to a larger target area and implantation deeper in the right ventricle, further away from atrial myocardium.19
The differences between both pacing strategies have been recently reviewed.20 A recent European survey demonstrated that most physicians with expertise in HBP were planning to start LBBAP; however, only 18% of physicians with expertise in LBBAP were planning to start HBP.21
Tools and Implant Techniques to Achieve Left Bundle Branch Area Pacing in Patients with Heart Failure
Implant techniques have been amply described and are beyond the scope of this article. However, when performing LBBAP in patients with HF, several things should be considered:
A lower LVEF has been associated with increased procedural complexity and lower procedural success rate, in part because severe dilatation in right-sided cardiac chambers can hinder lead drilling into the interventricular septum (IVS).22,23 Thus, it is important to obtain vascular access as proximal as possible (i.e. through an axillary or subclavian puncture, avoiding a cephalic cut-down technique). In certain cases, insertion of the LBBAP sheath inside a modified outer coronary sinus (CS) sheath (i.e. a sheath that has been cut down to an appropriate length) can facilitate sheath manipulation and proper placement against the IVS. Some of the sheaths used during LBBAP (Biotronik Selectra, Boston Scientific SSPC, Abbott Locator) offer larger curve models, which may be useful to engage for CCS patients with severely dilated right atria (RA), in whom the use of sheaths with smaller primary curves may lead to placing the sheath tip within the RA, and hinder crossing the tricuspid valve and/or facilitating lead dislodgement into the RA during sheath manipulation.
LBBAP has been performed with CRT pacemakers, CRT defibrillators, single and dual chamber pacemakers, and ICDs.24 Pacemakers have been used in lieu of traditional CRT pacemakers, apparently with good results, as the RV depolarisation will be provided by the right bundle branch (RBB). More recently, DF-1 ICDs have been used. By connecting the LBBAP lead to the IS-1 RV pace/sense port, CRT can be achieved without differences in successful arrhythmia detection.25 The use of standard defibrillator leads placed in the left bundle branch (LBB) area using non-dedicated transeptal sheaths has also been described.26 However, the lead and sheaths are not specifically designed for LBBAP, and the procedure is time-consuming, requiring multiple adjustments.
Currently Accepted Criteria for Left Bundle Branch Area Pacing
LBBAP includes LBB pacing (LBBP), which includes pacing of the main LBB or the left fascicles, and left ventricular septal pacing (LVSP); these different pacing targets can be identified using ECG-based criteria.
Left Bundle Branch Pacing
Successful LBB capture has been described in as many as 82–84% of patients with HF.27,28 There are several criteria to assess capture of the left CCS; all of them are highly specific, but have variable sensitivity (Table 1). These include:
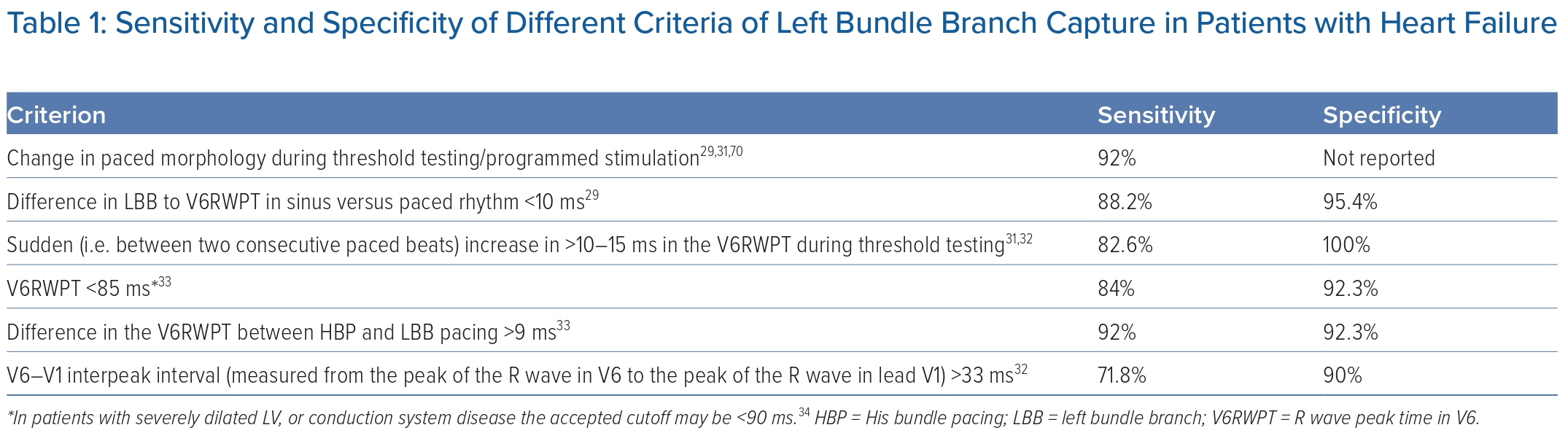
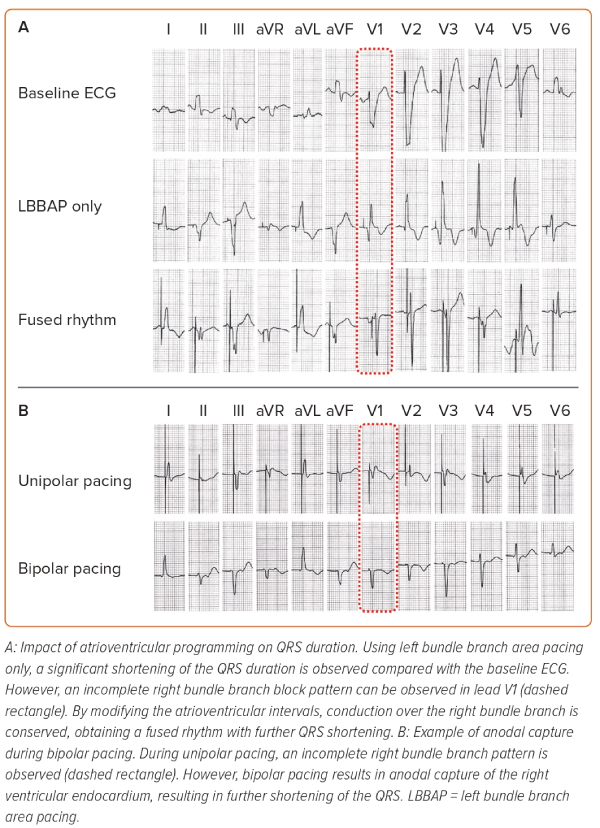
- Transition in QRS morphology, as capture transitions from non-selective LBB capture (observed in electrograms as a ventricular potential fused with the pacing stimulus) to either LVSP or selective LBBP (observed as the local ventricular electrogram occurring after a latency period following the pacing stimulus) during threshold testing/programmed stimulation.
- If an LBB potential can be observed during lead fixation, a difference between the LBB potential to R wave peak time in V6 (V6RWPT) during sinus rhythm and the stimulus–R wave peak time in V6 <5–10 ms is predictive of LBB capture.29,30 However, since most patients undergoing CRT have LBBB, this criterion is less useful.
- A sudden (between two consecutive paced beats) increase in >10–15 ms in the V6RWPT during threshold testing.31,32
- V6RWPT <85 ms (in patients with severely dilated LV or conduction system disease, the accepted cutoff may be <90 ms).33,34
- V6-V1 interpeak interval (measured from the peak of the R wave in V6 to the peak of the R wave in lead V1) >33 ms has a specificity of 90% (sensitivity 71.8%), while a value >44 ms has a specificity of 100%.32
In patients with LBBB, criteria for LBB capture have undergone less extensive evaluation. However, a change in QRS morphology (either during threshold testing or during programmed stimulation using extrastimuli) is observed in a significant proportion of patients.29 In patients with LBBB, non-specific interventricular conduction delay or escape rhythms, a V6RWPT <101 ms has been reported to have a 90.4% sensitivity and 78.9% specificity for LBB capture.29 A novel criterion using the transseptal conduction time, defined as the interval between the QRS onset and the first rapid change in the endocardial signal polarity obtained through the LBBB lead (measured within 15 ms of the beginning of the first notch or plateau in lead DI or augmented vector left), and the intrinsicoid deflection time, measured from the QRS onset to the beginning of the final downslope phase in lead V6 (or alternatively, in other lateral leads, such as DI, augmented vector left or V5), has been described. In patients with LBBB, the paced V6RWPT was >10 ms shorter than the difference between the intrinsicoid deflection time-transseptal conduction time in patients with LBB capture, but <10 ms in patients with LVSP. Using this cutoff, a sensitivity of 77.8% and a specificity of 100% for LBB capture in patients with pre-existent LBBB was observed.29
The recent consensus on conduction system pacing proposed a simple, stepwise approach using the following criteria:35
- QRS transition to LVSP or selective LBBP during threshold testing;
- V6RWPT <80 ms (in patients with LBBB);
- V6-V1 interpeak interval >44 ms; and
- QRS transition to selective LBBP during programmed stimulation.
If any of these criteria are met, the patient is said to have LBBP. If the patient has a V6RWPT <100 ms (in patients with LBBB), a V6-V1 interpeak interval >33 ms, transition to LVSP during programmed stimulation or QRS transition with a V6RWPT 10–14 ms during threshold testing, the patient is likely to have LBBP. In patients with a final R wave in lead V1, but without any of the above-mentioned criteria, LVSP is considered.
Left Ventricular Septal Pacing
Although LVSP does not produce direct activation of the CCS, by stimulating the left ventricular septum it eliminates the conduction delay associated with the electric impulse travelling across the IVS. LVSP results in significant reductions in the total ventricular activation time (i.e. the activation time of both ventricles) by producing near simultaneous activation of both ventricles, and is technically simpler than LBB pacing.36,37 However, when compared with LBB pacing, LVSP has been associated with significantly longer left ventricular activation time in V5-V6 and longer paced QRS durations.33,38 Consequently, this could potentially lead to a decrease in LV synchrony.39 In patients with HF, LBB pacing was associated with a significant reduction in the composite outcome of HF-related hospitalisation and all-cause mortality compared with LVSP (Cox proportional HR: 0.36, 95% CI [0.197–0.654]; p=0.001).40 Further studies are required to confirm these initial results.
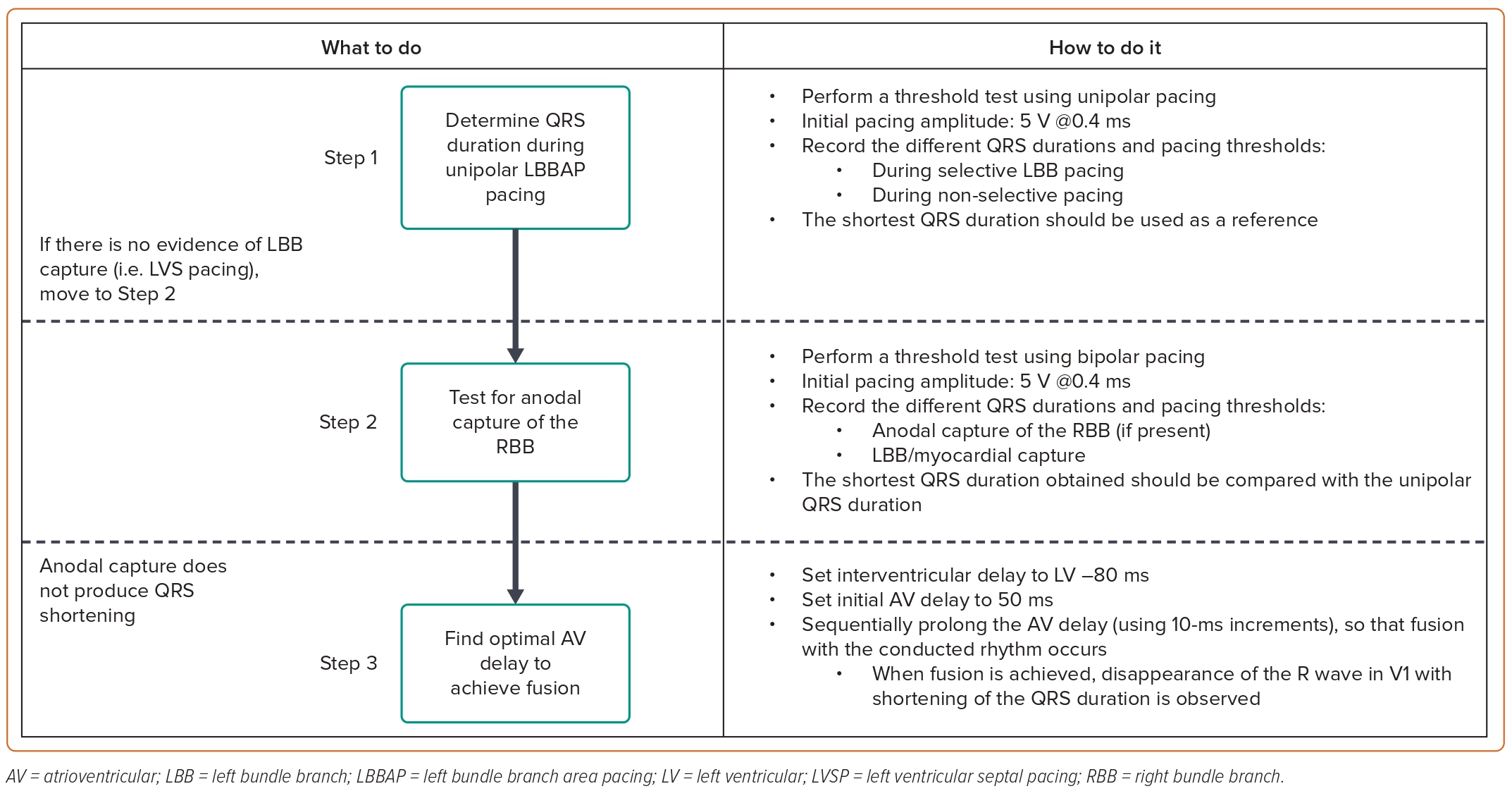
Device Programming in Patients with Heart Failure Undergoing Left Bundle Branch Area Pacing
During CRT, it is particularly important to achieve the shortest total ventricular activation time, as it correlates with improved haemodynamic responses.16 Our strategy to perform device programming is presented in Figure 1. First, QRS duration during unipolar pacing is measured at different pacing outputs, searching for the shortest QRS duration. Afterwards, bipolar pacing using different outputs is performed, as anodal capture of the RV can be achieved, resulting in QRS shortening.41,42 However, if achievement of anodal capture requires very high pacing outputs, it should not be used as it can result in early battery depletion. The selection between unipolar and bipolar pacing is then based on the shortest QRS duration. Atrioventricular (AV) delay is then programmed, aiming to achieve fusion between the paced LBBAP rhythm and the patient’s intrinsic conduction through the RBB, if present. Since the RA lead is generally placed in the RA appendage, very short AV intervals (sometimes as low as 50 ms) may be required to observe the R wave in lead V1, which should then be corrected with progressive prolongation of the AV interval. However, we program the AV interval 20 ms shorter than what is required to achieve complete correction of the pacing-induced RBB block. This ensures that during physical activity, which increases AV conduction speeds, enhanced conduction over the AV node and the RBB does not induce LBBB. When device programming is appropriately optimised to achieve simultaneous contraction of both ventricles, the interventricular synchrony with LBBAP can resemble the results of HBP (Figure 2).41 Finally, since there are so many different thresholds involved during LBBAP (i.e. LBB, septal myocardium, anodal RV myocardium), the device output should be programmed to capture the desired components to maximise the chances of improving interventricular synchrony.
Patient Follow-up
During follow-up, a small percentage of patients (4%) may experience loss of LBB capture.27,34,43 Therefore, it is recommended to perform a 12-lead ECG during each follow-up evaluation. This is particularly difficult to implement, as patients may require baseline and serial ECGs during programming. More recently, a novel method using a programmer ECG to evaluate a ‘pseudo-V1’ and a ‘pseudo-V6’ has shown a high correlation with measurements obtained from 12-lead ECGs.44 To achieve this, the right and left arm electrodes are positioned adjacent to each other at the right parasternal fourth intercostal space, while the right and left leg electrodes are placed adjacent to each other at the left fifth intercostal space along the midaxillary line, adjacent to one another. The pseudo-V1 lead is obtained by assessing lead DI in the programmer, while the pseudo-lead V6 is obtained by assessing lead DII/III in the programmer.
Evidence Supporting the Use of Left Bundle Branch Area Pacing in Heart Failure
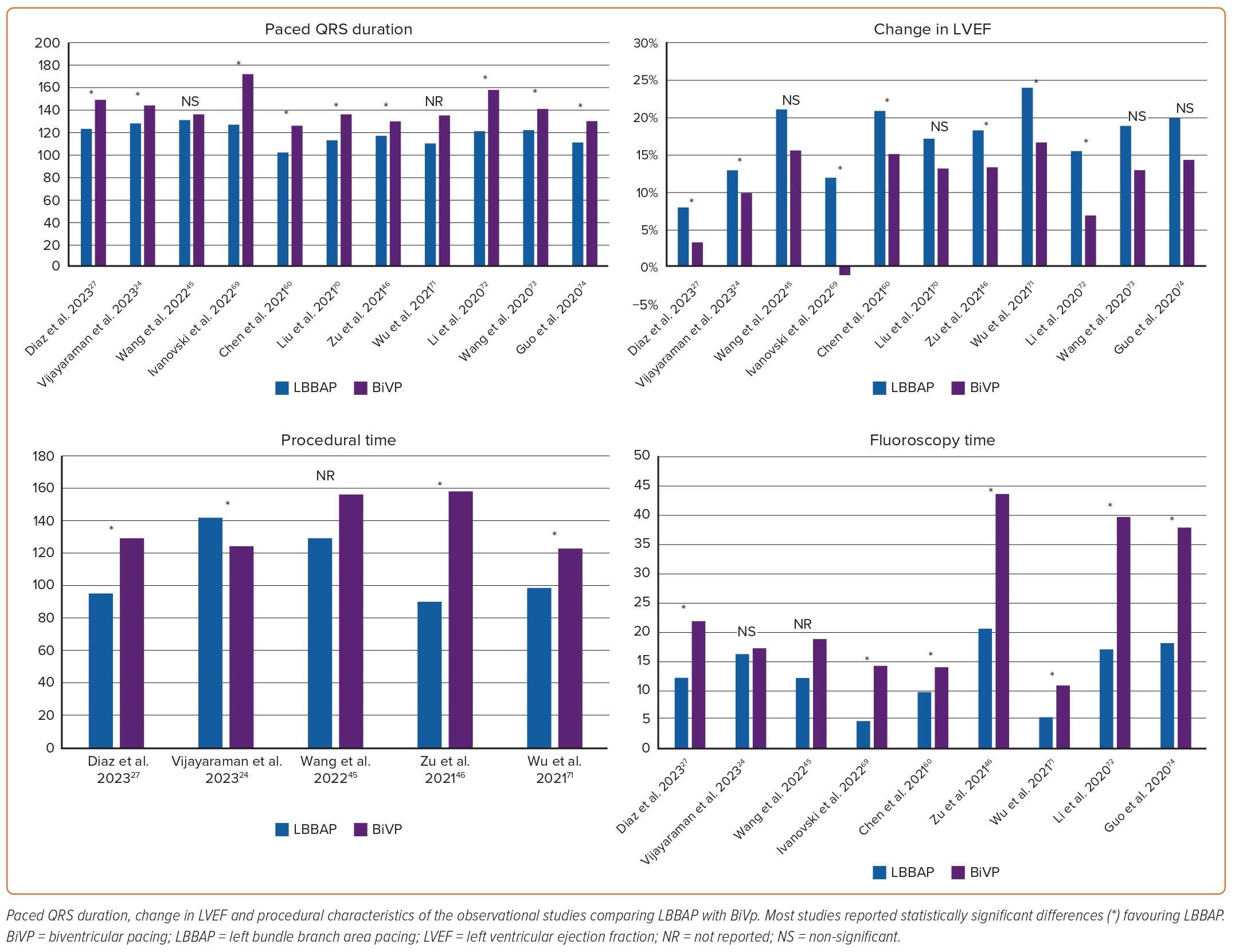
Despite the lack of randomised clinical trials directly comparing clinical outcomes between BiVp and LBBAP in patients with HF, several observational studies have compared these two CRT strategies (Figure 3, Supplementary Table 1). The use of LBBAP has been associated with a lower rate of HF-related hospitalisation (RR 0.60; 95% CI [0.39–0.93]; p=0.02; I2 0%), greater reductions in paced QRS duration (mean weighted difference 30.26 ms; 95% CI [26.68–33.84]; p<0.001; I2 13%) and improvements in LVEF (mean weighted difference 5.78%; 95% CI [4.78–6.77]; p<0.001; I2 0%) compared with BiVp. Moreover, the rate of echocardiographic response (defined as an absolute increase in LVEF by ≥5% between baseline and follow-up) and super-response (defined as an absolute increase in LVEF ≥20% or LVEF ≥50%) is significantly higher in patients undergoing LBBAP compared with BiVp (88.5 versus 72.5%; p=0.002; and 60.8 versus 36.5%, p<0.001, respectively).45
In a small randomised clinical trial, patients treated with LBBAP demonstrated greater improvements in LVEF compared with BiVp (mean difference 5.6%; 95% CI [0.3–10.9]; p=0.039).46 LBBAP has additionally demonstrated a significant reduction in procedural and fluoroscopy time.47 Notwithstanding, several factors have been identified as predictors of procedural failure during LBBAP. These include the presence of HF (OR 2.75; 95% CI [2.1–3.6]; p<0.001), larger LV diastolic diameter (OR 1.85; 95% [CI 1.59–2.16]; p<0.001 per 10-mm increase), baseline QRS morphology (OR 2.38; 95% CI [1.78–3.19]; p<0.001 for LBBB, intraventricular conduction delay or bifascicular block) and wide QRS.34 Thus, the use of LBBAP for CRT may require more expertise than that required for pacing in patients without structural heart disease.
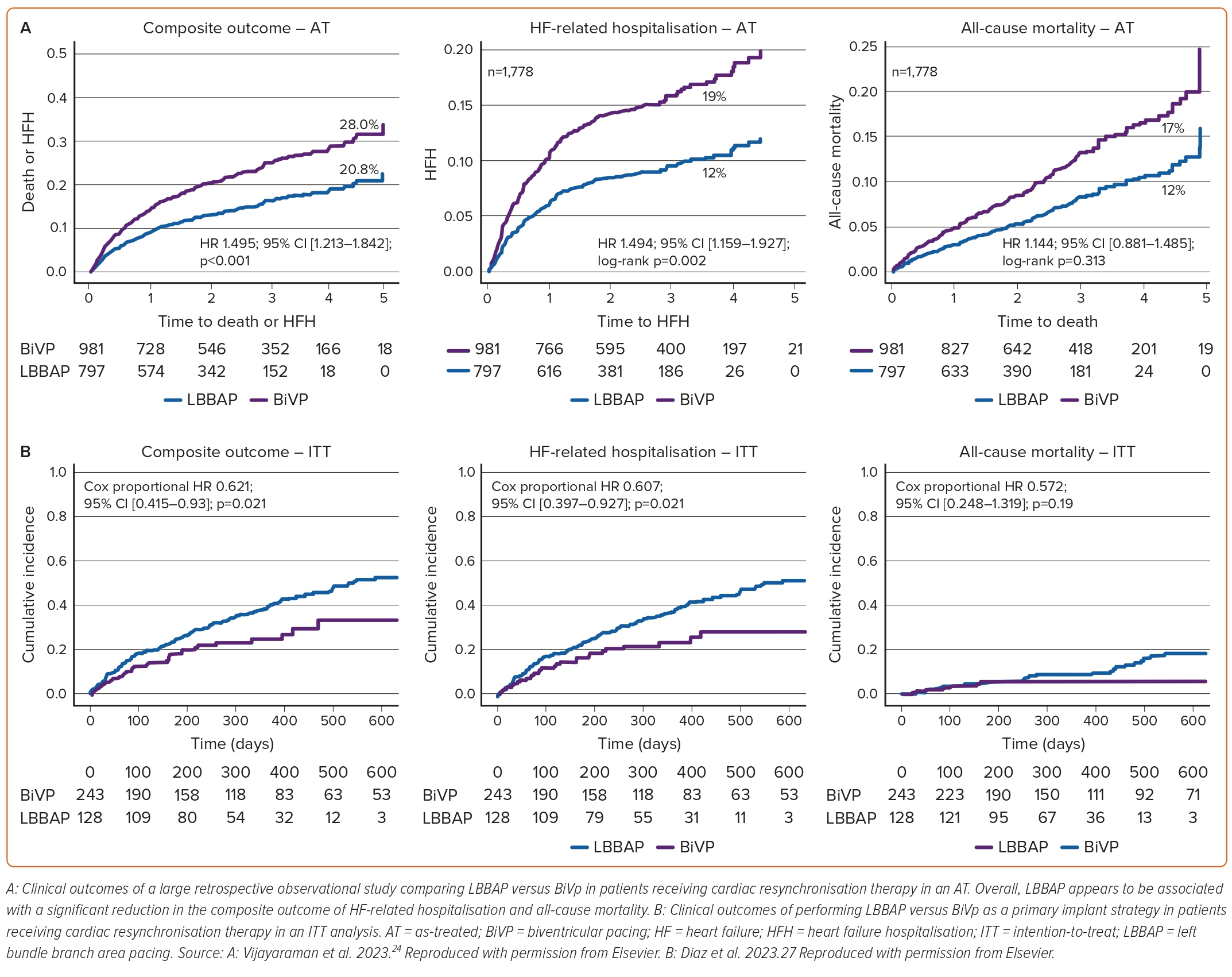
Vijayaraman et al. published a retrospective analysis of 1,778 patients undergoing BiVp (n=981) or LBBAP (n=797) for symptomatic HF with LVEF ≤35% and LBBB, or an expected RV pacing >40%, constituting the largest study evaluating the use of LBBAP in patients with HF to date.24 After a mean follow-up of 33 ± 16 months, BiVp was associated with a significant increase in the primary outcome, a composite of HF-related hospitalisation and all-cause mortality (HR 1.495; 95% CI [1.213–1.842]; p<0.001), without significant differences in all-cause mortality (Figure 4A). Interestingly, in this study, LBBAP was associated with significantly longer procedural times (142 ± 55 min versus 124 ± 48 min, respectively; p<0.001) without significant differences in fluoroscopy time (17 ± 15 min versus 16 ± 12 min, respectively; p=0.20). The reasons for these findings are unclear, and could be attributed to differences in operator expertise with LBBAP, or inclusion of a significant number of patients undergoing left bundle branch optimised CRT (LOT-CRT). LBBAP was associated with shorter paced QRS durations (128 ± 19 ms versus 144 ± 23 ms, respectively; p<0.001) and greater improvement in LVEF (15.3 ± 12% versus 10.8 ± 12%, p<0.001) compared with BiVp.
Acute Resynchronisation and Haemodynamic Response
LBBAP results in a greater shortening of the LV depolarisation time compared with BiVp (48.9 ± 12.5 ms versus 79.2 ± 13.1 ms; p<0.05), leading to a greater degree of intraventricular and interventricular synchrony (as indicated by a reduction in total ventricular activation time).48 Moreover, patients undergoing LBBAP have been reported to exhibit a significant improvement in the acute haemodynamic response, determined by the maximum rate of LV pressure rise, compared with patients undergoing BiVp.49
Left Bundle Branch Area Pacing in Patients Heart Failure and Mildly Reduced Left Ventricular Ejection Fraction
In patients with HR and mildly reduced LVEF associated with pacing-induced cardiomyopathy or intraventricular conduction anomalies, the use of LBBAP seems to be associated with a significant improvement in LVEF and a reduction in QRS duration.50 Unfortunately, there are no direct comparisons between LBBAP and other pacing strategies (HBP, RV pacing or standard BiVp) in this group of patients.
Left Bundle Branch Area Pacing as a Bailout Strategy or in Non-responders to Biventricular Pacing
Patients who have undergone an unsuccessful BiVp attempt with conventional CS leads usually receive a surgically implanted epicardial lead, and less frequently, HBP. The use of LBBAP as a bailout strategy results in significant reductions in QRS duration (166.7 ± 27 to 136 ± 26; p<0.001), improvement in functional status, and LVEF (29.2 ± 9.3% to 41.7 ± 11.9; p<0.001).51
Among non-responders to BiVp CRT or patients with CS lead failures, the use of LBBAP is associated with significant reductions in QRS duration (150 ± 22 versus 181.9 ± 26; p<0.001), as well as a significant increase in LVEF (26.7 ± 8 to 32.8 ± 9.6%; p<0.001). HF hospitalisations were lower in those with lead failure compared with non-responders (HR 0.357; 95% CI [0.168–0.756]; p=0.007). These results support the use of LBBAP as a reasonable bailout strategy for BiVp patients, as well as an alternative strategy for patients who are unresponsive to traditional BiVp.
Left Bundle Branch Area Pacing as an Initial Cardiac Resynchronisation Therapy Strategy
Recently, we published our experience with LBBAP as a primary implant strategy for patients with HF.27 In this prospective observational study, a total of 371 patients with symptomatic HF, either presenting with a LVEF ≤35% and LBBB, or LVEF ≤40% with an expected RV pacing >40%, underwent CRT. Among them, 243 patients received BiVp, while 128 patients underwent LBBAP. During a median follow-up of 340 days (IQR 206–477), patients who underwent LBBAP demonstrated a significantly lower incidence of the primary outcome, a composite of HF-related hospitalisation and all-cause mortality, than patients undergoing BiVp (HR 0.621; 95% CI [0.415–0.93]; p=0.021). This reduction was primarily driven by a significant reduction in HF-related hospitalisation (HR 0.607; 95% CI [0.397–0.927]; p=0.021), while there was no significant difference in all-cause mortality (Figure 4B). Additionally, LBBAP was associated with a significant reduction in fluoroscopy time (12 min [IQR 7.4–21.1] versus 21.7 min [IQR 14.3–30]; p<0.001), procedural time (95 min [IQR 65–120] versus 129 min [IQR 103–162]; p<0.001) and paced QRS duration (123.7 ± 18.8 ms versus 149.3 ± 29.1 ms; p<0.001) compared with BiVp. Use of LBBAP was also associated with a greater improvement in LVEF (8.04 ± 9.9% versus 3.9±7.9%; p<0.001) and a higher rate of patients experiencing improvement in at least one New York Heart Association class (80.4 versus 67.9%; p<0.001).
Although the external validity of the benefits associated with LBBAP in the treatment of HF is limited by the observational nature of the studies, the small number of patients included and the short follow-up, they provide a solid foundation for considering LBBAP during CRT, even as a first line implant strategy. The substantial improvement observed during a short follow-up suggests that LBBAP has the potential to exert a profound impact on HF-related outcomes. Moreover, as LBBAP has not been associated with a higher risk of procedure-related complications, it may soon be the primary implant strategy, despite the lack of randomised clinical trials.21
Left Bundle Branch Optimised Cardiac Resynchronisation Therapy
Vijayaraman et al. described a novel approach named ‘LOT-CRT’, which combines LBBAP and BiVp.52 This is performed by placing a standard LV lead, which is connected to the LV port, and an LBBAP lead, which is connected to the RV port in a DF-1 device. The success rate of this strategy was initially reported to be 81%, mainly limited by the inability to perform LBBAP.14 However, a more recent study reported a success rate of 96.8%.53
The use of a LOT-CRT strategy is associated with significantly shorter QRS durations, higher LVEF, and a lower incidence of a composite outcome of HF-related hospitalisations and all-cause mortality compared with BiVp.14,53 These results support the use of a LOT-CRT approach for patients in whom LBBAP alone does not result in a significant reduction in paced QRS duration, owing to distal LBBB or non-specific intraventricular conduction delay.
Complications Associated with Left Bundle Branch Area Pacing
Although penetration through the IVS is required, LBBAP is a safe pacing strategy, with a low rate of procedural complications:
Acute septal perforation occurs in 0.65% of procedures, while septal perforation during follow-up occurs in 0.33% of patients.54 Evaluating the amplitude of the current of injury in the tip electrode versus the ring electrode can be useful in detecting microperforation: a ratio <1 has a sensitivity of 100% and a specificity of 96.6%.55 In most cases, intraprocedural septal perforation does not require any additional studies or interventions. In these cases, the lead should be withdrawn, the sheath repositioned at a new location and the lead deployed again.
Lead dislodgement occurs in 7–12% of patients, and appears to be more common with the use extensible helix (adjusted HR 2.86; 95% CI [1.15–7.13]; p=0.024).27,34,56
Lead fracture can occur intraprocedurally (with an estimated incidence of 0.33%) or during follow-up.54 Additionally, lead-to-lead interaction has been reported as a cause of lead failure; avoiding contact between the RV lead and the LBBAP lead may reduce this risk.57
IVS haematoma has been rarely described, and should be suspected when patients present with chest pain and elevated troponins after LBBAP procedures.58–60 Conservative treatment can be implemented with resolution of the haematoma over the next months; in some cases, coiling of the ruptured artery may be necessary.
Intraprocedural RBB injury is generally transient and recovers before the end of the procedure.61 Importantly, RBB injury in patients with LBBB can result in complete heart block, and the operator should be mindful, preparing for it accordingly.
Coronary artery injury, most frequently to the first septal perforator, has been described. In most instances, conservative management is recommended, as the size of the shunt is minimal and has no haemodynamic consequences. The septal perforators arising from the left anterior descending artery are longer (40–80-mm long) and more numerous than those originating from the posterior descending artery (15 mm).62,63 Thus, targeting the inferior IVS could potentially reduce the risk of septal coronary artery laceration (Figure 5).
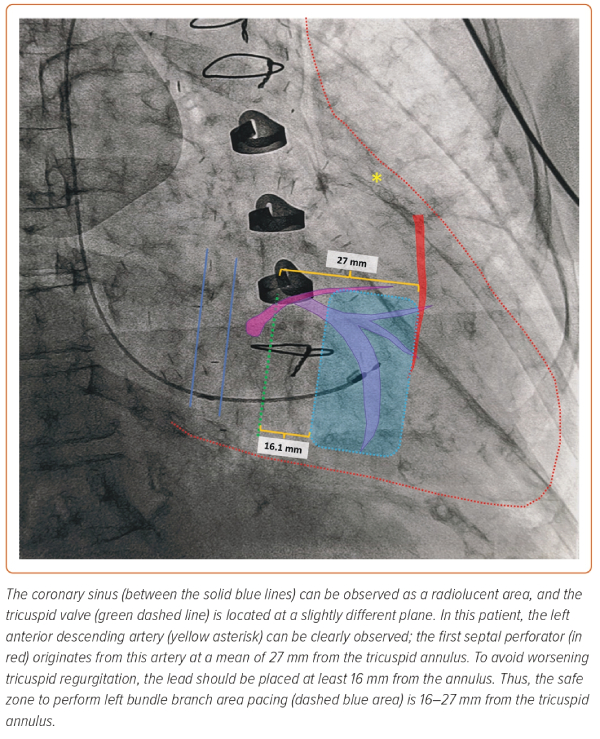
Importantly, the first septal perforating artery is the largest septal perforator, and its course is closely related to the pulmonary valve, approaching the subpulmonary infundibulum immediately below the two pulmonary sinuses adjacent to the aorta.63 It is located at a mean distance of 27 mm from the His bundle in direction towards the RV apex, with a distance >20 mm in 84% of patients.64 Although lesions to the left anterior descending artery have been described, to produce a lesion to this artery, the lead has to be inserted in a very superior and anterior position, which is not recommended and easily avoided with a good right anterior oblique fluoroscopic projection.65
Worsening tricuspid regurgitation due to fixation of the septal valve or the chordae tendineae can occur as the LBBAP lead is driven into the IVS. Placing the LBBAP lead at a distance >16.1 mm from the tricuspid annulus significantly reduces this risk.66 Importantly, the risk of tricuspid regurgitation during LBBAP does not appear to be different from conventional RV septal pacing; however, more data is required.67
Future Studies
Currently, there are several randomised trials evaluating the impact of LBBAP versus BiVp on HF-related outcomes, including the following.
The LeCaRt trial (NCT05365568) will randomise 170 patients with LBBB and QRS duration >130 ms or any intraventricular conduction delay and QRS duration >150 ms to either LBBAP or BiVp. The primary outcome is a composite of death, hospitalisation or unscheduled visit for HF or worsening HF symptoms, with adaptation of the medical therapy, implant failure for any cause and implantable electronic cardiac device re-intervention for any reason during follow-up. The estimated completion date is September 2024.
The LEFT-BUNDLE-CRT trial (NCT05434962) will randomise 176 patients with a class I or IIa indication for CRT and LBBB according to the Strauss criteria to either LBBAP or BiVp. The primary outcome is a positive CRT response, defined either by an improved clinical composite score or ≥15% reduction in LV end-systolic volume. The estimated completion date is December 2024.
The RAFT-P&A trial (NCT05428787) will include 284 patients with atrial fibrillation considered for AV node ablation for rate control and a baseline N-terminal prohormone of brain natriuretic peptide >600 or >400 if HF hospitalisation occurs within 12 months. They will be randomised to AV nodal ablation + BiVp CRT versus AV nodal ablation + LBBAP. Interestingly, no specific cutoff for LVEF is specified in the trial design. The primary outcome will be the change in N-terminal prohormone of brain natriuretic peptide from baseline to 6-month follow-up. The estimated completion date is July 2024.
The Left versus Left trial (NCT05650658) will randomise 2,136 patients with a resting QRS duration >130 ms (with no specific mention of LBBB) or an expected RV pacing >40% and a LVEF ≤50%. The primary outcome is the combined clinical endpoint of all-cause mortality and HF-related hospitalisation. The estimated completion date is June 2029.
Current Guidelines
A clinical consensus statement on conduction system pacing implantation was recently published, including general definitions, a summary of the available evidence and tools and techniques to achieve both HBP and LBBAP.35 This document provides a valuable summary on the available evidence surrounding LBBAP. Additionally, the most recent HRS/APHRS/LAHRS guideline on cardiac physiological pacing for the avoidance and mitigation of HF consider LBBAP as a reasonable alternative to BiVp for patients in whom effective CRT cannot be achieved (class 2a recommendation).68 Moreover, guidelines provide a class 2b recommendation for LBBAP as an alternative to BiVp, particularly for operators with previous experience with this technique.
Areas of Uncertainty
Given that LBBAP is a relatively new technique and there are limited data in HF patients, long-term follow-up data on clinical outcomes are lacking. Current available data are based on observational studies, thus being inherently prone to selection bias. Therefore, the true magnitude of the impact of LBBAP on HF-related outcomes will not be fully established until data from randomised clinical trials is available. The increasing adoption of LBBAP as a CRT strategy has been driven by expert opinion based on available observational evidence and physiological understanding of CCS pacing. Furthermore, there are concerns regarding lead fracture during long-term follow-up, since leads were not purposefully designed to stand the mechanical stress at the hinge point produced by insertion of the lead deep into the IVS. Nonetheless, available studies assessing lead performance estimated the risk of lead fracture over a span of 10 years to be 0.02%, which is comparable with observed fracture rates for RV pacing leads.69
Conclusion
LBBAP is a novel strategy to achieve CCS pacing. By targeting the LBB area, it enables effective capture of the distal CCS in most patients, leading to significant improvements in LV synchrony. As a result, there is a growing interest in the use of LBBAP in patients with HF as a strategy to achieve CRT. The currently available evidence, primarily derived from observational studies, consistently demonstrates substantial benefits associated with LBBAP. These benefits include a significant improvement in LVEF, along with reduced QRS durations, shorter procedural and fluoroscopy times, along with a reduction in HF-related hospitalisations. The benefit seems to sustain when LBBAP is used either as a bailout strategy to conventional BiVp or as a primary implant strategy. Further randomised clinical trials are needed to determine the impact of LBBAP therapy in HF patients. 
Click here to view Supplementary Material
Clinical Perspective
- Left bundle branch area pacing (LBBAP) achieves capture of the cardiac conduction system in a significant proportion of patients, with low and stable pacing thresholds, adequate lead stability, and short procedural and fluoroscopy times.
- In patients with heart failure requiring CRT, LBBAP achieves significant improvements in interventricular and intraventricular synchronisation, as well as improving clinical outcomes.
- LBBAP has been shown to reduce the risk of heart failure hospitalisations, improve left ventricular ejection fraction and functional class, and achieve shorter QRS duration than biventricular pacing in patients with heart failure.