AF is the most common sustained arrhythmia, and catheter ablation of AF is one of the most common cardiac electrophysiological procedures.1 Despite recent advances in catheter ablation procedures, the recurrence rate of AF has remained considerably high. With the advent of techniques and devices such as contact force and ablation index, pulmonary vein isolation (PVI) durability can be increased.2 Nevertheless, the procedural duration was considerably long, thus imposing constraints on the quantity of ablation that can be performed in a given day, and thereby leading to a lengthy waiting list. Meanwhile, waiting time and longer time to ablation from diagnosis were associated with increased morbidity and AF recurrence.3,4
One of the current approaches is very-high-power short-duration (VHPSD) ablation, which can significantly shorten the procedure time.5–7 By increasing the power output it is possible to reduce the duration of ablation per lesion tag to 4–7 seconds, along with the potential creation of shallower but wider lesions, thus facilitating complete isolation while also reducing the procedure time. VHPSD catheter ablation (70–90 W, 4–7 seconds) has emerged as a method that is comparable or even superior to low-power long-duration (LPLD) ablation (30–40 W, >20 seconds) and standard high-power short-duration (HPSD) ablation (50 W, 7–11 seconds) in terms of effectiveness, even when compared with ablation index-guided procedures, while enabling a more expeditious procedure.5,8 Nevertheless, the use of the very-high-power ablation technique raises safety concerns. The aim of this systematic review and meta-analysis was to aggregate the latest evidence and compare the effectiveness and safety of VHPSD PVI with those of conventional ablation in patients with AF.
Methods
Literature Search Strategy
Two reviewers conducted an extensive literature search independently, via PubMed, SCOPUS and EuropePMC, using the specified keywords (((very-high-power short duration) OR (QDOT microablation)) AND (atrial fibrillation) AND (ablation)) up to 16 June 2023. The discrepancies that emerged were effectively addressed through discussions. The eligibility of the records was assessed using predetermined criteria for inclusion and exclusion.
The authors of this study performed a systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol of this systematic review is registered at PROSPERO (CRD42023437156). A flowchart of the process of the literature search is shown in Supplementary Figure 1.
Intervention and Control Groups
The intervention group (VHPSD) consisted of AF patients undergoing PVI using VHPSD catheter ablation, defined as a power of 70 W or more and a duration of 7 seconds or fewer. The control group (conventional) consisted of patients with AF undergoing PVI using conventional ablation (LPLD or HPSD ablation) using any ablation technique (including contact force and ablation index-guided ablation). In the VHPSD group the procedures were carried out using the QDOT MICRO ablation catheter (Biosense Webster) and FlexAbility Cardiac ablation Catheter, SE (Sensor Enabled; St Jude Abbott).
Our research protocol permits investigations involving both PVI alone and PVI with additional ablation beyond PVI, including but not limited to posterior wall isolation, substrate modification approach by complex fractionated atrial electrogram ablation and linear ablation, and cavotricuspid isthmus (CTI) ablation, in which additional analyses were conducted in relation to these procedures.
Selection Criteria
We included randomised controlled trials, observational studies (both prospective and retrospective) reporting the incidence of AF recurrence in VHPSD catheter ablation, and studies comparing its efficacy with conventional ablation. We excluded animal studies, letters to editors, supplementary journals, review articles, case reports, and non-English language articles.
Outcomes
The primary outcome of this study was AF recurrence, which is defined as AF, atrial flutter or atrial tachyarrhythmias that persists for more than 30 seconds at least 3 months after ablation.
The secondary outcomes were procedure duration (minutes), fluoroscopy duration (minutes), radiofrequency ablation time (minutes) and complications (i.e. complications related to the procedure, such as vascular access, and complications related to catheter ablation, such as pericardial effusion and/or tamponade, oesophageal injury and/or fistula, cerebrovascular accident, pulmonary vein stenosis, MI, thromboembolic events and steam pops).
Data Extraction
The process of data extraction was conducted by two reviewers separately using a form detailing primary outcome, other outcomes, study design, VHPSD methods, conventional ablation methods, sample size, catheter used, population included, interlesion distance (mm), paroxysmal AF (percentage), onset from diagnosis, repeat ablation, additional ablation beyond PVI, follow-up duration, age, male sex, left atrial size (volume and diameter), left ventricular ejection fraction (LVEF), hypertension, diabetes, congestive heart failure, follow-up modality and timepoints, and detailed complications. The risk of bias in the studies was independently assessed by two reviewers, using the Newcastle–Ottawa Scale for observational studies and the Cochrane risk of bias for randomised controlled trials. Any disagreements that arose during the process were resolved by discussion.
Statistical Analysis
This meta-analysis was performed using Stata 17. Meta-analysis of proportion was performed for each event per total. The fixed-effect method is used for conducting comparative analysis on continuous variables with the inverse-variance approach and on binary variables with the Mantel–Haenszel method. In cases in which significant heterogeneity is observed, the random-effects model, specifically the restricted maximum-likelihood (REML) approach, is used as an alternative. Statistically significant heterogeneity was defined as I2 >50% and/or a p-value for heterogeneity below 0.10. The pooled analysis was deemed statistically significant when the p-value was less than 0.05. The odds ratio was used to measure binary comparison, and mean difference was used to measure continuous variable comparison. Egger’s test was used to quantitatively measure small-study effects. Funnel-plot analysis followed by trim-and-fill analysis was used to qualitatively measure small-study effects and publication bias. A meta-regression analysis using the REML approach was conducted to examine the effect of moderating variables on the incidence and comparison of AF recurrence between the VHPSD and conventional ablation groups. This analysis focused on the baseline factors age, sex (male), hypertension, diabetes and the percentage of paroxysmal AF, and only variables that were reported by a minimum of 10 studies were analysed.
Sensitivity analyses were performed to exclude a follow-up duration of 3 months, and different catheter and power settings. Subgroup analysis was performed for additional ablation beyond PVI.
Results
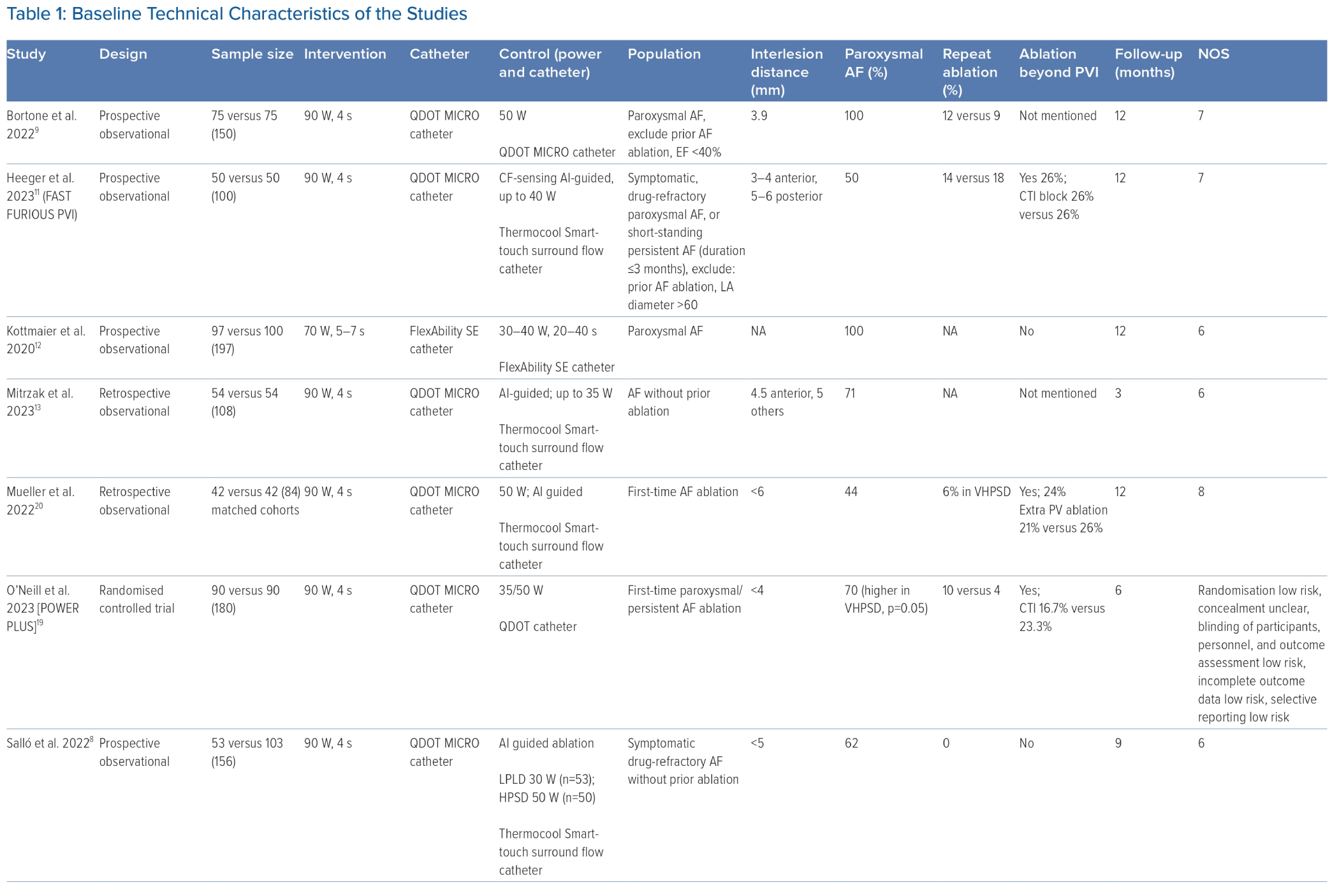
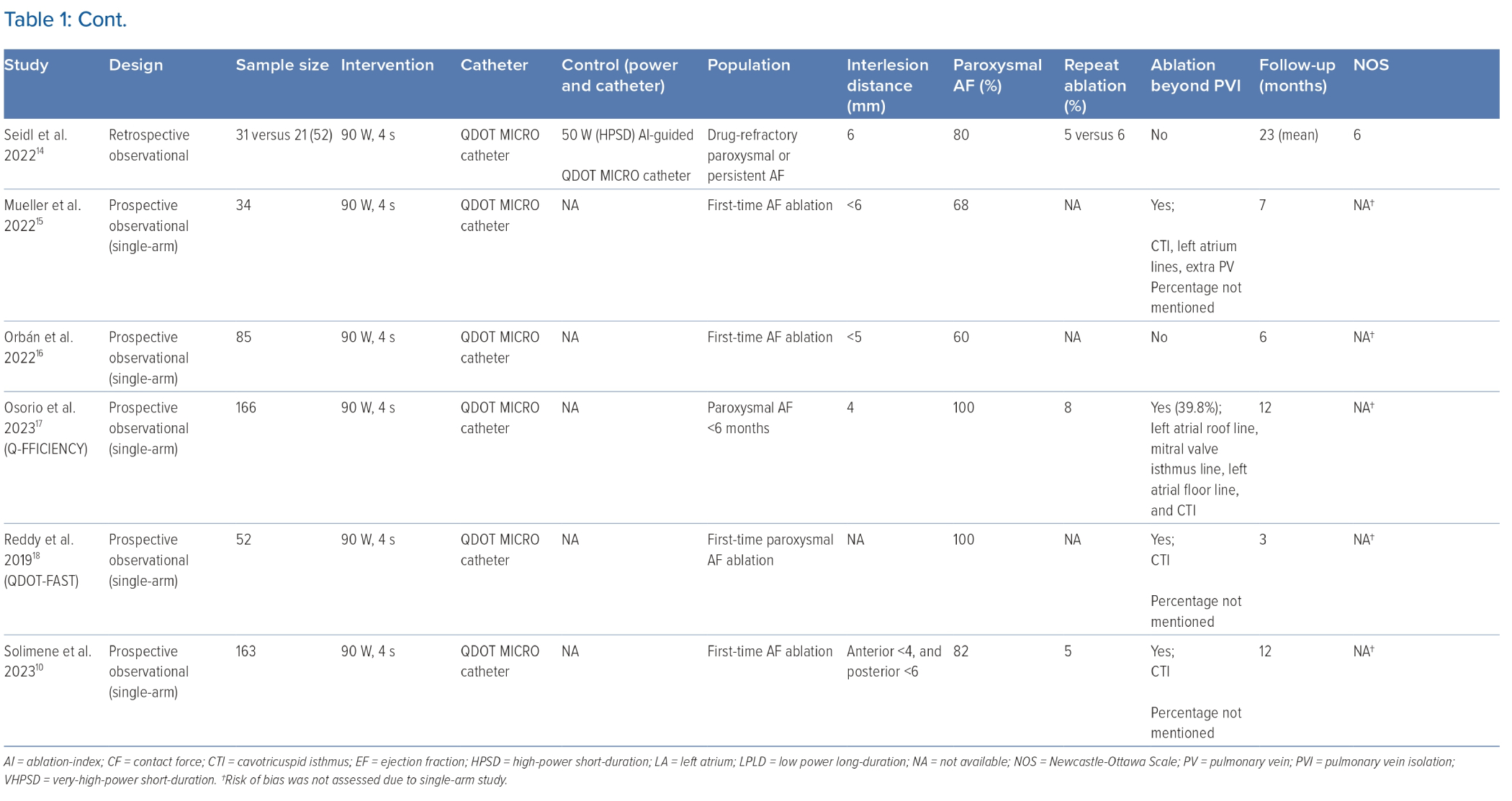
This systematic review and meta-analysis encompassed 13 studies with a sample size of 1,527 patients. Of these 13 studies, one was a randomised controlled trial, four were prospective observational studies, three were retrospective observational studies, and five were single-arm prospective cohorts.8–18 The baseline characteristics of the included studies are listed in Tables 1 and 2. All but one study used the QDOT MICRO catheter at 90 W and a 4-second ablation duration. Four studies reported only paroxysmal AF, whereas the other nine studies reported both paroxysmal and persistent AF. Seven studies performed additional ablation beyond PVI, four did not, and two did not mention it. The follow-up duration ranged from 3 months to 23 months after the procedure, in which Holter monitoring was performed after a 3-month blanking period.
Atrial Fibrillation Recurrence
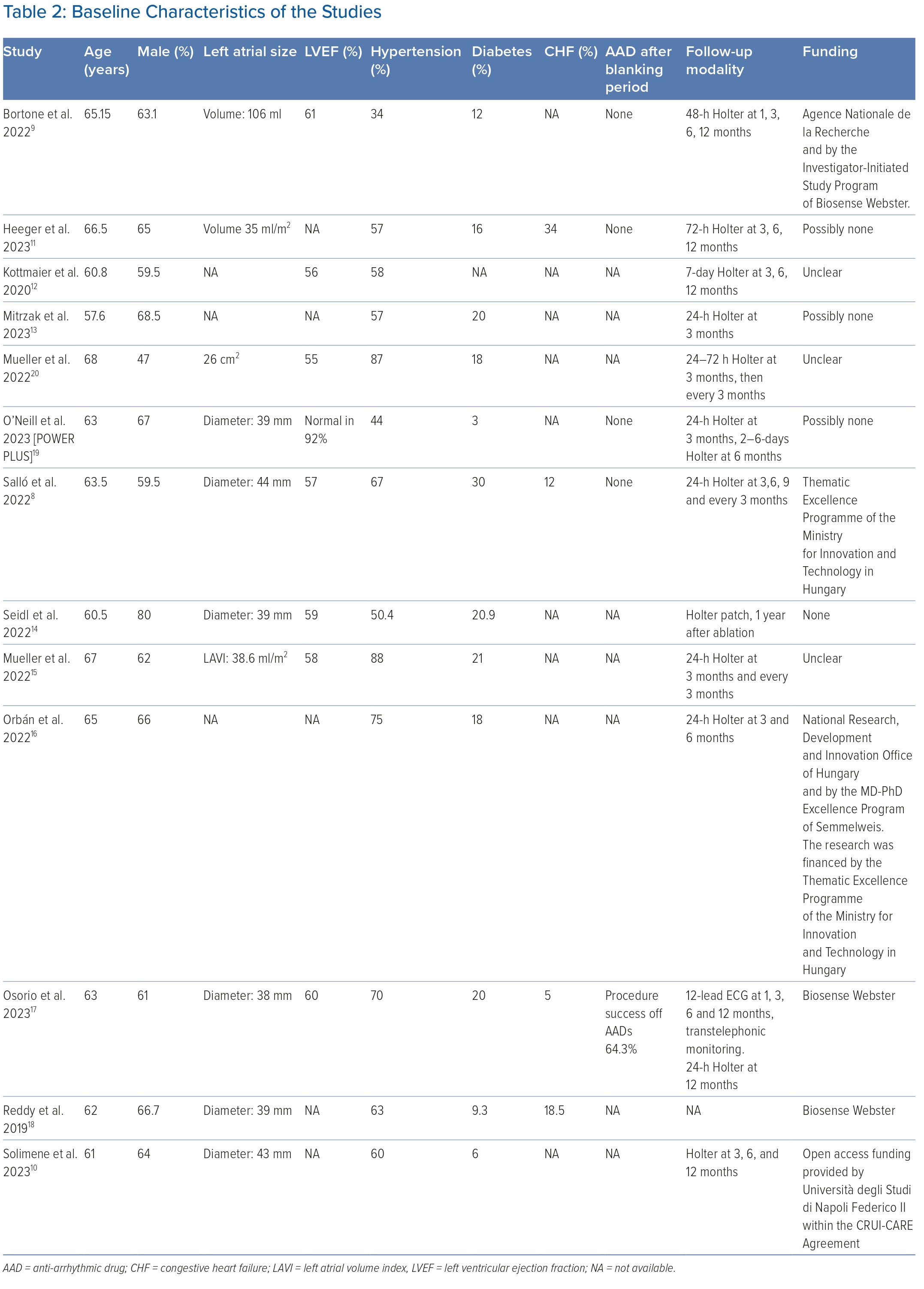
The mean duration of follow-up was 9.9 ± 5.1 months. AF recurrence was observed in 14% of the VHPSD group (95% CI [11–18%]; I2 66.7%; p<0.01; Figure 1A) and 24% of the conventional group (95% CI [16–32%]; I2 82.8%; p<0.01). The incidence of AF recurrence was not significantly modified by age (p=0.960), male sex (p=0.142), LVEF (p=0.695), hypertension (p=0.890), diabetes (p=0.919) or paroxysmal AF (p=0.294) on meta-regression analysis. On subgroup analysis the rate of AF recurrence was 16% (95% CI [10–22%]; I2 72.6%; p<0.01) in groups with additional ablation beyond PVI and 10% (95% CI [6–15%]; I2 39.4%; p=0.18) in groups without additional ablation beyond PVI. After performing sensitivity analysis that excluded studies with a follow-up period of 3 months, the rate of AF recurrence was 14% (95% CI [11–18%]; I2 61.7%; p<0.01).
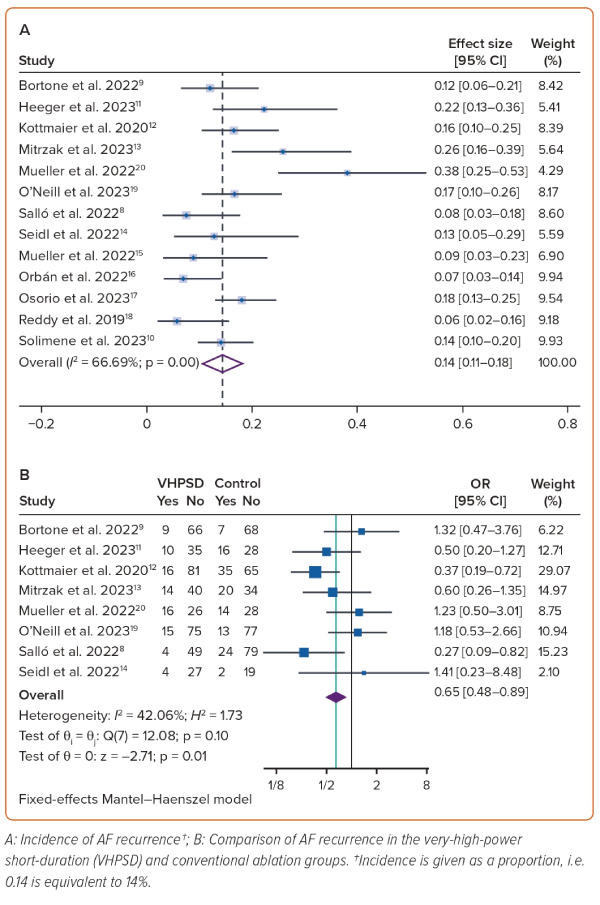
VHPSD had a lower AF recurrence rate (OR 0.65; 95% CI [0.48–0.89]; p=0.006; I2 42%; p=0.10) than the conventional ablation group (Figure 1B). Subgroup analysis of studies without additional ablation beyond PVI (n=3 studies) showed reduced AF recurrence in VHPSD compared with conventional ablation (OR 0.38; 95% CI [0.22–0.65]; p<0.001).12,14,19 Sensitivity analysis by excluding studies with a follow-up of 3 months yielded similar results (OR 0.66; 95% CI [0.47–0.92]; p=0.015; n=7 studies).8,9,11,12,15,19,20 However, sensitivity analysis involving the removal of the 70 W setting and ensuring that the catheters were the same in the VHPSD ablation group, resulted in no significant difference between the VHPSD and the conventional ablation groups (OR 0.77; 95% CI [0.54–1.09]; p=0.14; n=7 two-arm studies).
Procedure Time
Procedure duration was significantly shorter in the VHPSD group with a mean difference of −14.4 minutes (95% CI [−26.1, −2.6]; p=0.017; I2 83.3%; p<0.001; Supplementary Figure 2A). Fluoroscopy duration was similar in the two groups with a mean difference of −0.58 minutes (95% CI [−3.22, 2.07]; p=0.670; I2 94.3%; p<0.001; Supplementary Figure 2B). Radiofrequency ablation time was significantly shorter in the VHPSD group, with a mean difference of −14.1 minutes (95% CI [−14.10, −8.84]; p<0.001; I2 94.1%; p<0.001; Supplementary Figure 2C).
First-pass Isolation
First-pass isolation was achieved in 69% of the VHPSD group (95% CI [57–81%]; I2 90.7%; p<0.001) and 74% of the conventional ablation group (95% CI [65–84%]; I2 81.5%; p<0.001). There was no significant difference in the rate of first-pass isolation between the two groups (OR 0.63; 95% CI [0.33–1.20]; p=0.160; I2 70%; p=0.003).
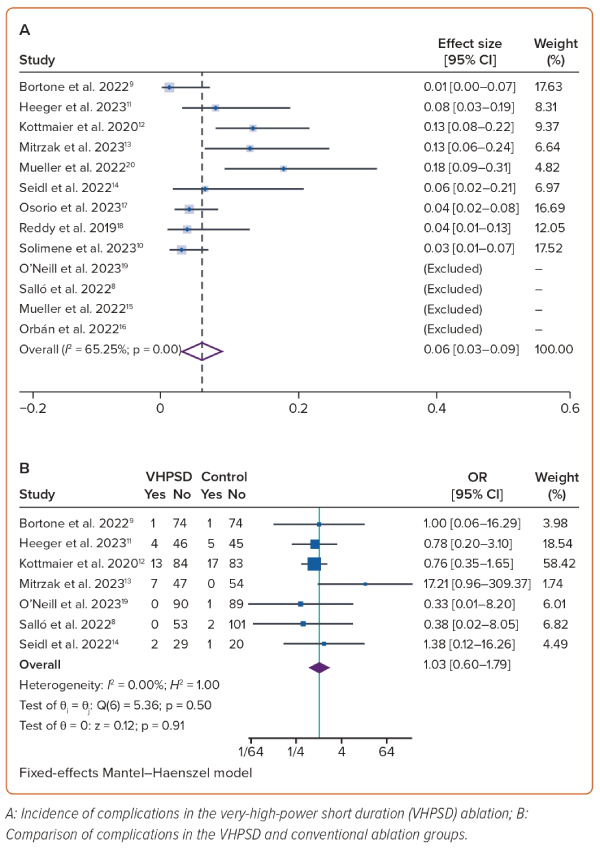
Complications
Complications occurred in 6% of the VHPSD group (95% CI [3–9%]; I2 65.3%; p<0.01; four studies cannot be included due to 0 events; Figure 2A).9–15,17,18 The rate of complications was similar in the two groups (OR 1.03; 95% CI [0.60–1.80]; p=0.498; I2 0%; p=0.907; Figure 2B). Most complications were related to vascular access, such as bleeding and groin haematoma (Table 3). The incidence of serious adverse events related to catheters, such as effusion and tamponade, was reported in 6/349 patients (1.7%) and 3/873 patients (0.3%), respectively.8,9,11–15,17,18,21 There was no incidence of atrio-oesophageal fistula, or clinically apparent or endoscopically detected oesophageal injuries. Coagulation formation of catheter tip was seen in 18% of the patients in a study by Mueller et al.; Mitrzak et al. reported a cerebrovascular accident rate of 1.85% (one patient), and Reddy et al. reported an asymptomatic microembolism rate of 1.9% (one patient).13,15,18 The other seven studies reported zero incidence of transient ischaemic attack, stroke, or other thromboembolic events. No mortality was reported in any study.
Publication Bias
The Egger’s test results suggest that there is no evidence of small-study effects on the efficacy of VHPSD ablation compared with conventional ablation in terms of AF recurrence (p=0.299). The results of the funnel-plot analysis indicated a distribution that was largely symmetrical, as shown in Supplementary Figure 3. In addition, the trim-and-fill analysis indicated that no studies could be imputed.
Discussion
This is the first meta-analysis to compare VHPSD with conventional ablation (including HPSD), which also demonstrates that additional ablation beyond PVI may not be required. VHPSD ablation is associated with a lower rate of AF recurrence and with a shorter procedure duration compared with conventional ablation methods. The incidence of complications was low in both the VHPSD and the conventional ablation procedures.
The use of VHPSD ablation resulted in a 35% decrease in AF recurrence compared with conventional ablation with low heterogeneity. VHPSD ablation creates a shallower and wider lesion compared with conventional ablation, thus resulting in better encirclement and 100% lesion contiguity compared with only 25% in LPLD.21,22 The application of high-power ablation in an animal model produced a reduction in both macroscopic and microscopic gaps.20,22 Evaluation of left atrial scars on MRI showed durable and transmural PV lesions with homogeneous and contiguous scar formation at follow-up.23
The duration and stability of catheter contact play crucial roles in the effective delivery of radiofrequency energy. Using VHPSD it is possible to decrease the required contact time. However, the presence of catheter instability can be a limitation in VHPSD ablation. This is due to the relatively short contact time of only 4 seconds, which means that any movement of the catheter during this period can have a more pronounced impact on the quality of the lesion, as opposed to LPLD ablation.9 In instances when the operator encounters such difficulties, the operator may switch to the conventional method. Heeger et al. performed VHPSD ablation with a very-CLOSE protocol (inter-lesion diameter of 3–4 mm anteriorly and 5–6 mm posteriorly) based on the wider but shallower lesion depth of VHPSD compared with conventional ablation.11
This systematic review notes that there was no significant difference in terms of cardiac perforation in VHPSD compared with conventional ablation. The use of VHPSD ablation can be particularly advantageous in cases involving thin atrial tissues, because it facilitates the creation of shallow and wide lesions. Therefore, our results challenge the concerns that higher power delivery is related to a higher risk of tamponade. However, in instances when the tissues are thicker, a combined approach involving conventional ablation techniques may be necessary to obtain optimal results.17 However, Heeger et al. showed that VHPSD ablation can be used without resorting to conventional ablation methods.11 Salló et al. noted a lower AF recurrence in VHPSD ablation than conventional ablation despite not adjusting the power based on the position of the lesion.8
The subgroup analysis indicates that the incidence and benefit of VHPSD in reducing AF recurrence were similar for patients who did and those who did not undergo additional ablation beyond PVI. There is limited evidence to support the use of additional ablation techniques beyond PVI, such as posterior wall isolation, substrate modification using complex fractionated atrial electrogram ablation, linear ablation, and prophylactic CTI ablation, given that they have not demonstrated significant clinical advantages.24–27 This meta-analysis supports the findings of these studies, and additional ablation may not be necessary. However, further studies that directly compare VHPSD ablation with and without additional ablation beyond PVI are needed to validate this finding.
Sensitivity analysis was performed to exclude studies with a follow-up duration of 3 months, which produced similar results to those for VHPSD ablation. However, when the sensitivity analysis excluded studies with different power settings and catheters, there was no significant difference in terms of AF recurrence between the VHPSD and the conventional ablation groups.
As expected, the procedure and the radiofrequency time were notably reduced in patients who underwent VHPSD ablation compared with those who received conventional ablation, which can be even shorter without additional ablation beyond PVI. There was no significant difference in fluoroscopy time because practitioners who are proficient at ablation with a 3D mapping system tend to use fluoroscopy sparingly during ablation; thus, the time was similar between the two groups.
In terms of safety, it is noteworthy that VHPSD ablation has a relatively low incidence of adverse events, and these are primarily attributed to vascular access complications, which are not directly linked to the use of the high power setting. Other complications, such as pericardial effusion (reported in six patients) and tamponade (reported in three patients), were rare and comparable to conventional ablation. The studies did not report oesophageal lesions necessitating intervention, however, caution should be exercised to avoid the overlapping of VHPSD lesions, particularly in thin areas near the oesophagus.17 Coagulation formation on the catheter tip is not uncommon; nonetheless, it did not result in clinically apparent adverse events.15
Nevertheless, a significant proportion of individuals who undergo VHPSD ablation experience silent cerebral events.15 Due to the absence of preprocedural cerebral MRI as a comparator, there is uncertainty regarding the extent of procedure-related silent cerebral events in patients with AF, who are already susceptible to such events. The majority of silent cerebral lesions are asymptomatic and resolve on their own; effective management of sheaths, minimising left atrial dwell time, continuing anticoagulant, maintaining a target activated clotting time above 300 seconds, and delaying postablation cardioversion for 4 weeks may reduce neurological adverse events.28 In the long term, the benefit of sinus rhythm restoration in terms of cerebrovascular events outweighs the risk of catheter ablation.28–31 Halbfass et al. reported no complications of steam pop, cardiac tamponade, stroke, oesophageal ulceration or fistula, or catheter charring on the nMARQ generator used in Bruges.32 The QDOT MICRO ablation catheter (Biosense Webster) enables temperature- and flow-controlled ablation, thus enabling larger, shallower, more homogeneous, and fewer haemorrhagic lesions.33
Limitations
There is a lack of randomised studies investigating VHPSD versus conventional ablation. The follow-up duration of the studies was short; therefore a longer follow-up is required to evaluate PVI durability. Most of the studies used a QDOT MICRO catheter, with only one study using a FlexAbility Cardiac Ablation Catheter, SE; thus, the results cannot be generalised to other catheters. The heterogeneity of the conventional ablation group due to the different power and duration settings, which generate different lesion characteristics and clinical outcomes, may affect the results. The meta-regression analysis may be underpowered due to the limited number of studies. The inability to analyse some of the variables in the meta-regression may confound the results. The included studies did not uniformly report variables such as left atrial diameter or volume, and anti-arrhythmic drugs management. Anti-arrhythmic drug management may affect AF recurrence. Time from onset or from diagnosis to ablation is an important prognostic factor that most studies have not reported.4 The underlying substrate in AF may be heterogeneous; thus, additional specific lesions may require additional intervention.
Conclusion
VHPSD ablation is associated with lower AF recurrence and a shorter procedure time than conventional ablation. Furthermore, additional ablation beyond PVI in VHPSD may not provide additional benefits in addition to PVI alone. Cardiac complications were rare in both the VHPSD and the conventional ablation groups; the complications were mostly attributed to vascular access, and there was no significant difference between the two groups. 
Click here to view Supplementary Material
Clinical Perspective
- Very-high-power short-duration (VHPSD) catheter ablation is associated with lower AF recurrence and shorter procedure time than conventional ablation.
- Routine additional ablation beyond pulmonary vein isolation (PVI) in VHPSD may not provide additional benefits compared with PVI alone. Additional ablation may be considered in individual cases with typical atrial flutter and in patients with recurrent AF after the initial PVI or in patients who cannot maintain stable sinus rhythm despite successful redo PVI.
- Cardiac complications were rare in both VHPSD and conventional ablation, and consisted mostly of vascular access complications.