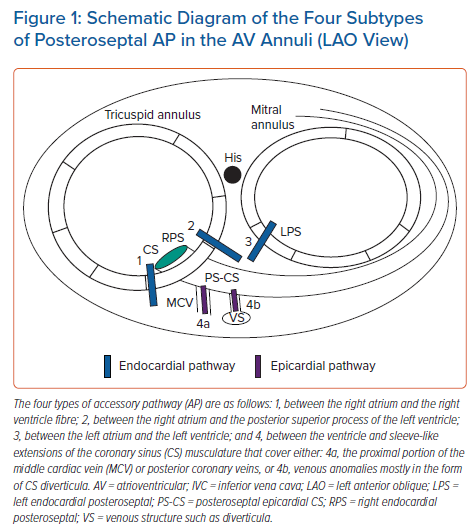
Radiofrequency catheter ablation has become the preferred treatment option for patients with symptomatic Wolff-Parkinson-White (WPW) syndrome or recurrent symptomatic orthodromic reciprocating tachycardia. The success of the procedure depends on the accurate localisation of the accessory pathway (AP). In that respect, posteroseptal or inferior paraseptal APs, which represent the second most common atrioventricular (AV) connection site after left free wall AP, often pose a diagnostic challenge. This reflects the complex anatomy at the crux of the four cardiac chambers, where a small area may encompass APs that may be approached from the right or left endocardium, or require an ablation performed epicardially inside the coronary sinus (CS).
APs located in the posteroseptal area can take a variety of courses. Four different course types may be distinguished (Figure 1):
- Endocardially between the inferior paraseptal right atrium and the right ventricle. This area includes the inferior part of the Koch’s triangle and the area surrounding the CS ostium.
- Endocardially between the inferior paraseptal left atrium and the left ventricle.
- Coursing between the inferior paraseptal right atrium and the left ventricle in the pyramidal space, given that the right atrium lies directly on the posterior superior process of the left ventricle. This anatomical conformation results from the fact that the interatrial septum lies leftward to the interventricular septum and the tricuspid annulus is displaced 5–10 mm apically with respect to the mitral annulus.1 The right atrial endocardial aspect overlying the posterior superior process of the left ventricle lies between the most posterior aspect of the right fibrous trigone and the CS ostium, medial to the tricuspid valve. Because of its close proximity, ablation of these APs may be possible from the proximal CS. Based on past surgical experience, an important subgroup of inferior paraseptal APs is actually right atrial to left ventricular connections.2,3
- Epicardially, connecting the musculature overlying the CS to the ventricle. These connections are probably most often related to sleeve-like extensions of the CS musculature that cover the proximal portion of the middle cardiac vein or posterior coronary veins. In 70% of these APs the CS venous anatomy is normal, while in the remaining cases venous anomalies are identified, mostly in the form of CS diverticula.4 Most of these APs are ablated with a coronary venous approach but it is worth noting that a successful ablation within the CS does not imply a CS musculature-related connection but may merely reflect the proximity of the CS to atrioventricular muscle connections.5 Here, these APs are referred to as ‘epicardial CS’ APs.
Because of the anatomical complexity of the inferior paraseptal region and the fact that APs may overlap or extend in adjacent areas, these APs often shared the lowest ablation success rate with right free wall APs in previous series.6,7 Similarly, a high recurrence rate has been reported, in the range of approximately 10%.6–8 In that respect, one must be aware that APs ablated within the coronary venous system present a much higher risk of developing slow conduction and decremental properties after an ablation attempt compared with other localisations.9 Finally, the need for a percutaneous subxiphoid approach in inferior paraseptal APs has more often been reported in case reports or series, possibly because of ablation limitations for CS musculature-related AP.10
The procedural risks of inferior paraseptal AP ablation will notably differ depending on whether a left-sided approach or a CS ablation is required, mainly as a consequence of the risk of embolisation or damage to coronary artery, respectively.4,11–14 Considering these differences, an accurate anticipation of the precise location of inferior paraseptal AP is critical to inform the discussion and consent process with the patient and to guide the mapping strategy. Here, we will review the clues to discriminate APs that can be ablated from the right atrium from those requiring a left-sided or epicardial coronary venous approach. Both manifest and concealed APs will be considered and, following the diagnostic process made by the operator before starting the interpretation of the intra-cardiac signals, each of the following aspects will be addressed:
- clinical context and initial probability; and
- 12-lead ECG analysis during baseline ECG with manifest AP, maximal pre-excitation and orthodromic reciprocating tachycardia.
Clinical Context and Initial Probability
The proportion of inferior paraseptal APs that can be ablated from the right atrium or that require a left-sided or epicardial CS approach has varied in previous reports for a number of reasons. First, the distinction between left inferior paraseptal and left posterior AP is ill-defined when considering ablation procedures performed under fluoroscopic guidance only. From an anatomical standpoint, the left boundary of the inferior paraseptal region is 2.3 ± 0.5 cm distant from the CS orifice.15 Second, studies that have included the so-called epicardial CS APs in their analysis often considered together both left endocardial inferior paraseptal and left posterior APs.4,16 Finally, the proportion of APs requiring an epicardial coronary venous approach is dependent on the ablation strategy. In our practice, radiofrequency application within the CS is never performed before ruling out a possibly successful ablation on the left endocardial side in order to limit the risks of complications such as coronary artery damage.13,14 This strategy may potentially underrepresent the prevalence of epicardial CS APs compared with an ablation strategy performing CS ablation more liberally.
When left inferior paraseptal and epicardial CS APs are defined based on a successful ablation from 7 to 8 o’clock along the mitral annulus, and ≥1 cm within the CS (including its proximal branches), respectively, then the majority of inferior paraseptal APs (in the range of 50–60%) can be ablated from the right side.17–21 Epicardial APs requiring a coronary venous approach represent approximately 10–20% of inferior paraseptal APs, while the remaining APs can be successfully ablated on the septal mitral annulus.4,16,19–21
Two notable exceptions to this AP distribution are worth mentioning: the Ebstein’s anomaly and the permanent form of reciprocating tachycardia caused by slowly conducting bypass tracts. In Ebstein’s anomaly, the vast majority of inferior paraseptal APs are ablated on the anatomical tricuspid annulus.22–24 Regarding APs exhibiting the phenotype of permanent junctional reciprocating tachycardia, approximately three-quarters are located in the inferior paraseptal region (range, 50–88%).25–28 Of these, 80–100% can be successfully ablated with an exclusive right-sided approach, mostly around the CS ostium. The remaining cases are ablated within the CS or its proximal branches, or on the left septal mitral annulus.25,26,28 APs with Mahaim conduction characteristics will not be considered in this review because most of them originate at the lateral aspect of the tricuspid annulus. However, it is worth noting that inferior paraseptal locations may also be found.29
The 12-Lead ECG Analysis
The first step of the diagnostic process is based on the 12-lead ECG analysis of either the ventricular preexcitation pattern or, for concealed APs, the retrograde atrial activation during orthodromic reciprocating tachycardia. The preexcitation pattern may be analysed during baseline ECG or during maximal preexcitation, such as in antidromic reciprocating tachycardia, preexcited AF, adenosine infusion or during rapid atrial pacing manoeuvres performed during electrophysiological studies.
Baseline ECG Analysis in Manifest Accessory Pathway
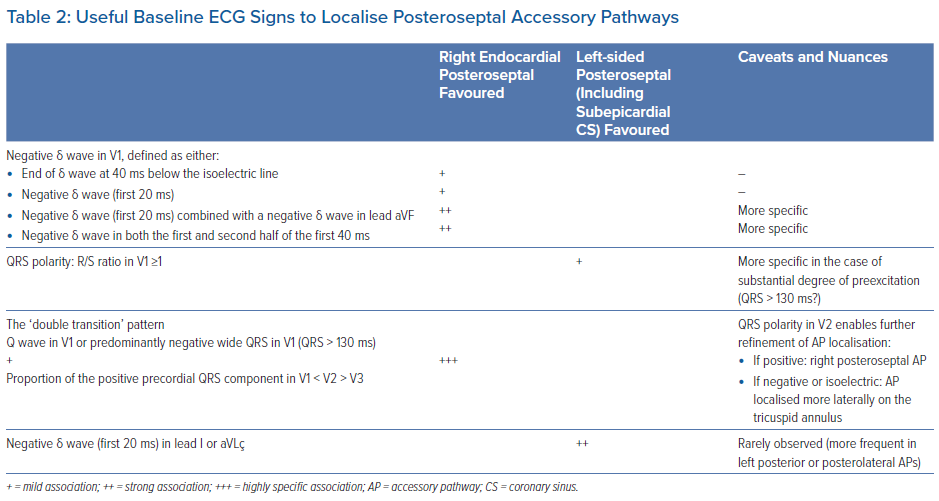
Several algorithms based on the delta wave and/or QRS polarity have been previously proposed to characterise the AP localisation in WPW patients.17,20,21,30–36 However, their accuracy is limited mainly by the fact that the QRS complex is a fusion of varying degree with the activation from the His–Purkinje system. The interpretation is also flawed due to a number of other factors, such as the variations in body shape and/or size, the heart’s location and anatomical characteristics. Finally, up to 10% of patients may have more than one AP, which can make the ECG difficult to interpret. Therefore, these criteria provide only an approximate indication and attempts to distinguish localisations that are only 1–2 cm apart should be interpreted cautiously. Their accuracy is especially modest for certain locations, such as the inferior paraseptal region, considering the complex local anatomy as discussed previously.37
Regarding the specific characterisation of inferior paraseptal APs, the goal of most algorithms was to discriminate the laterality between right or left endocardial localisations. Only a limited number of studies have included in their analysis bypass tracts requiring ablation performed within the CS.4,17,19,20,27,38 These algorithms have considered either the QRS polarity or the delta wave polarity, the latter being generally measured from the onset of the earliest delta wave observed in any of the peripheral leads. Direct comparison between studies is limited by the fact that the analysis of the delta wave polarity substantially differed between studies (see below).
The most common characteristics that have been proposed by authors to distinguish inferior paraseptal APs from other localisations can be summarised as follows:
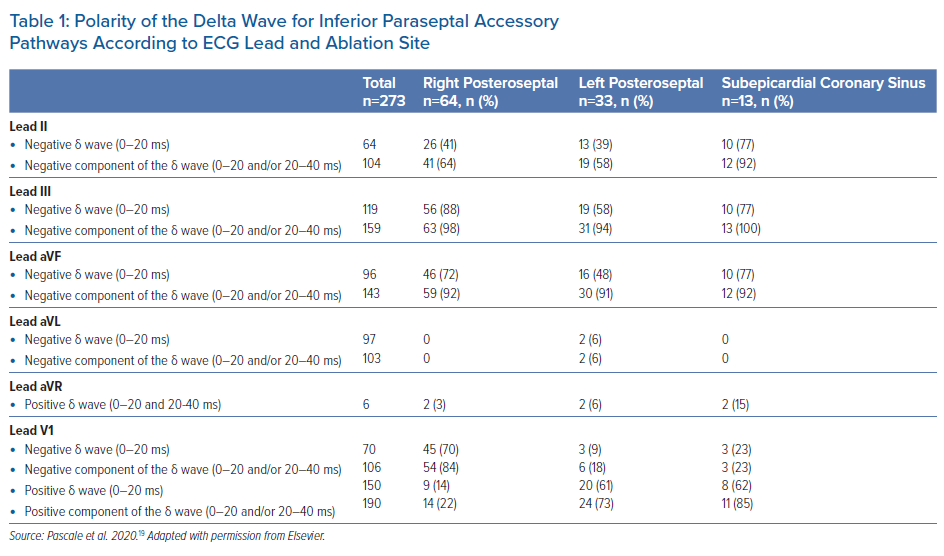
- Negative delta waves in at least two inferior leads, given that the delta wave in lead II less often displays a negative delta wave, and may be isoelectric or biphasic.17,19,21,27,31–33 The delta wave polarity was variably defined: some studies considered the initial 20 ms, and some the initial 40 ms.17,33,31,32 Isoelectric delta waves were also considered together with negative delta waves.17,31,33 In our practice, we rather consider both the first and the second half of the 40 ms period, and would suspect an inferior paraseptal AP when a negative component is observed in either period.19 We compared this method with the assessment of the initial 20 ms only.19 As illustrated in Table 1, considering only delta waves whose initial 20 ms are negative, the sensitivity to detect inferior paraseptal APs was notably reduced.
- A positive delta wave in leads I and aVL in order to distinguish them from left posterior/posterolateral APs (or ‘left free wall’ APs), which may display a negative delta wave.17,19,27,31,33 In our experience, a negative component of the delta wave (first 20 ms), or a fully isoelectric delta wave (40 ms), may sometimes be observed in left endocardial inferior paraseptal APs, but we did not observe this pattern in right-sided or epicardial CS APs (Table 1).
- An early precordial lead transition. To distinguish inferior paraseptal APs from ‘right free wall’ APs, which generally display later precordial transitions. These right free wall APs comprise right posterior or posterolateral APs, but also right lateral bypass tracts given that the latter sometimes also display negative delta waves in inferior leads. This early transition was variably defined by authors as an R/S ratio >1 in V2;31,34 or an RS or Rs QRS pattern in V1–V3.33
Identification of Epicardial Coronary Sinus Accessory Pathway
Regarding the differential diagnosis of inferior paraseptal APs, there have been various attempts to specifically identify epicardial CS APs ablated within the CS based on a standard sinus rhythm ECG.17,19,20 The most widely reported pattern was first described by Arruda et al.17 They suggested that the identification of a negative delta wave in lead II was specifically associated with epicardial CS APs (100% sensitivity and specificity). Their stepwise ECG algorithm was based on the polarity of the initial 20 ms of the delta wave and the first step consisted in ruling out a left free wall AP based on the delta wave polarity in leads I and V1. However, this association between a negative delta wave in lead II and epicardial CS APs should by no means be regarded as definitive.
Indeed, in a later study involving almost 10-fold more epicardial CS APs, Arruda’s group reported a much lower sensitivity, with 70% of epicardial CS APs displaying a negative delta wave in lead II.4 Regarding the specificity of this association and its reproducibility in other study populations, we found that a negative delta wave in lead II was indeed significantly more often observed in epicardial CS APs compared with right or left inferior paraseptal APs.19 However, the specificity was limited considering that 35–40% of endocardial inferior paraseptal (left and right), left posterior and right free wall APs all displayed a negative delta wave in lead II. Accordingly, when applying the stepwise diagnostic algorithm proposed by Arruda et al. in our population, the specificity and positive predictive value (PPV) of a negative delta wave in lead II to predict epicardial CS APs were only 68% and 18%, respectively.17,19 The sensitivity was 77%, which compares well with the aforementioned findings reported in the later study by Arruda’s group.4
Takahashi et al. also attempted to identify APs ablated within the CS in a selected population of 117 patients with manifest inferior paraseptal AP.20 They evaluated the initial 40 ms of the delta wave and also found that negative delta waves in lead II were more often observed in APs ablated within the CS compared with those ablated from the right or left endocardium (87% versus 21%, p<0.01). The sensitivity of that finding was high in their cohort (87%). However, similar to the results in the study by Pascale et al., the specificity and PPV of this association were low considering that the study population was a selected one, consisting of only inferior paraseptal APs (79% and 50%, respectively).19,20 Takahashi et al. also observed that a positive delta wave in lead aVR was more often observed in epicardial CS APs compared with right or left inferior paraseptal APs (57% versus 9%, p<0.01). In that selected population the specificity and PPV of this finding were 91% and 62%, respectively. The reproducibility of this result in the Pascale et al. study cannot be precisely assessed. The finding of a positive delta wave in lead aVR, both in the first and second half of the 40 ms period, was indeed specific for AP localised in the inferior paraseptal region, but it was rarely observed in the population from the Pascale et al. study: it was observed in only 15% of epicardial CS APs compared with 3% and 6% in right and left inferior paraseptal APs, respectively (p=0.07 and 0.31, respectively) (Table 1).19
In summary, there seems to be little evidence to support the fact that a specific ECG pattern enables selective discrimination of epicardial CS APs from other APs based on a standard sinus rhythm ECG. Considering the limitations in the ECG interpretation discussed above, the identification of such a specific ECG pattern seems unrealistic.
It seems therefore more realistic to aim for the identification of features that are specific to APs located at some distance from the overlapped endo- and epicardial components of the left atrium. As such, we think that an approach aiming to discriminate inferior paraseptal APs ablated from the right endocardium from left-sided APs (endocardial or epicardial CS) is most reasonable. This distinction is the most relevant in terms of procedural risk anticipation and procedural planning, given that a left-sided approach would be advised even if an epicardial CS AP is initially suspected.
Right Endocardial Posteroseptal versus Left-sided Posteroseptal Accessory Pathway
Based on our experience and previous data, there is no single ECG sign that allows to discriminate the laterality of most inferior paraseptal APs. Nevertheless, there are some ECG features based on the delta wave or QRS polarity that enable localisation of the subgroups of inferior paraseptal APs with a reasonable specificity. By summing the knowledge of these different criteria, the categorisation of a substantial proportion of APs may be achieved.
Delta Wave in the Frontal Plane
Regarding the delta wave polarity in the frontal plane, we were not able to find any specific pattern able to discriminate a significant number of right and left inferior paraseptal APs (Table 1).19 Similarly, Haghjoo et al. found that the delta wave polarity in the inferior leads could not distinguish right from left inferior paraseptal APs.21
In contrast, some authors suggested that the combination of a positive initial delta wave in lead II (0–20 ms) with a negative delta waves in leads III and aVF was specific for right inferior paraseptal AP given that it points towards a more right-sided location.33 In our experience, this pattern seems indeed specific for right endocardial septal AP but it is rarely observed, given that less than 10% of patients with inferior paraseptal AP had this pattern.19 Of note, as mentioned before, the delta wave, considered as a whole, is often ‘less negative’ in lead II in inferior paraseptal APs whether right- or left-sided.
The finding of a negative delta wave in all inferior leads has also been regarded as suggestive of a right endocardial AP, generally related to the CS orifice region.33 In our study population this finding provided the exact same information as a negative delta wave in lead II with respect to the AP localisation, given that all of those patients also had a negative initial delta wave in leads III and aVF.19 As such, this pattern instead suggested an epicardial CS AP and was also often observed in left endocardial APs (36%), as discussed before.
Finally, as mentioned above, when an inferior paraseptal AP is suspected, the finding of a negative component of the delta wave (first 20 ms) in leads I or aVL essentially rules out a right-sided, and possibly also an epicardial CS AP. Nevertheless here, again, this finding was rarely observed in left endocardial inferior paraseptal APs (Table 1).19
Accordingly, the delta wave polarity in the frontal leads seems to be of limited value to discriminate right versus left-sided APs.
Delta Wave in Precordial Leads
Regarding the delta wave polarity in the precordial leads, there are conflicting data concerning the assessment of V1 to distinguish between right- versus left-sided APs. As discussed before, these discrepancies may in part be related to the method used to define the delta wave polarity.
In their study assessing the initial 40 ms of the delta wave in patients with manifest inferior paraseptal AP, Takahashi et al. found that a negative delta wave in V1 was present only in right endocardial APs (28% versus 0%, p<0.01).20 A negative polarity was considered when the end of the delta wave was below the isoelectric line. In contrast, Haghjoo et al. evaluated the polarity of the initial 60 ms of the delta wave and found no statistically significant difference between the proportion of positive, negative and biphasic delta waves in V1 when comparing right endocardial, left endocardial and epicardial CS inferior paraseptal APs.21 In that study, polarity was considered negative or positive when the delta wave was entirely below or above the isoelectric line, respectively. In contrast to these findings, despite using the same delta wave polarity definition, Chiang et al. had previously reported that a positive delta wave in V1 could specifically differentiate left from right inferior paraseptal APs.31 In our previous study, we tested different ways of assessing the delta wave polarity including a separate assessment of the first and second half of the first 40 ms of the delta wave.19 As shown in Table 1, a negative initial delta wave (0–20 ms) pointed towards a right endocardial inferior paraseptal AP with a fair specificity. This pattern was observed in 70% of right inferior paraseptal APs, while only 9% and 23% of the left inferior paraseptal and epicardial CS APs had a negative initial delta wave, respectively (p<0.01 and p=0.001, respectively).
Similar findings were observed when the delta wave polarity defined by Takahashi et al. was applied to the population in the study by Pascale et al.: 69% of the right inferior paraseptal APs had a negative delta wave in V1 while only 12% and 15% of the left inferior paraseptal and epicardial CS APs had a negative delta wave, respectively (p<0.01 for both comparisons).19 Arruda et al. showed that right inferior paraseptal APs could be more specifically identified by combining the finding of a negative, or isoelectric, initial delta wave in V1 with a negative delta wave in lead aVF.17
In the Pascale et al. study population, this pattern was fairly specific and provided 58% sensitivity, 93% specificity and 73% PPV.19 Its specificity could be further increased when only negative delta waves were considered in both V1 and aVF (45% sensitivity, 96% specificity and 78% PPV).19
In our experience, however, the most specific pattern is the finding of a negative delta wave in both the first and second half of the first 40 ms of the delta wave. This pattern was almost 100% specific for right endocardial AP and was observed in approximately half of the patients.19
Accordingly, lead V1 may provide some useful indication depending on how the delta wave polarity is defined.
Analysis of the QRS Polarity
Regarding the analysis of the QRS polarity, different authors have showed that an R/S ratio in V1 ≥1 is a sensitive and specific marker (up to 100%) to differentiate left from right inferior paraseptal endocardial APs (including the CS ostium).21,32,39 Of note, APs ablated from within the CS have not consistently been included in these analyses. However, it appears that even APs ablated from the most proximal part of the CS (<1–1.5 cm from the ostium) more often have an R/S ratio ≥1 in V1.1,18,21,39,40 Moreover, it may be anticipated that, the further from the CS ostium, the more likely it is that APs will have an R/S ratio in V1 ≥1. However, in a previous review, Haissaguerre et al. instead noted the value of an R/S ratio <1 in V1.40 In their experience, all inferior paraseptal APs with prominent negative QRS complexes in V1 were ablated from the right side (88% endocardially, 12% in the proximal CS). APs with prominent positive QRS complexes were ablated at the right endocardium, the proximal CS or left endocardium in 55%, 26% and 18% of cases, respectively.40
On the other hand, other authors did not find a significant yield of the R/S ratio to discriminate between right- and left-sided inferior paraseptal APs.31,33
We also evaluated the R/S ratio in V1 to discriminate right- from left-sided inferior paraseptal APs. We found that an R/S ratio ≥1 was significantly more frequent in left-sided APs: it was observed in 76% of left endocardial APs and in 69% of epicardial CS APs. Right endocardial APs had an R/S ratio ≥1 in 25% of cases (p<0.001 and p=0.002 compared to left endocardial and epicardial CS APs, respectively) (Pascale, 2021 unpublished data). What we observed on analysis of these data is that in the majority of cases of left-sided AP with a predominantly negative QRS complex in V1 there was in fact a weak degree of preexcitation with a relatively narrow QRS. Not surprisingly, this limitation is even more relevant when assessing the polarity of the QRS rather than that of the delta wave. This drawback may explain the differences between studies and must be kept in mind when assessing the R/S ratio in V1.
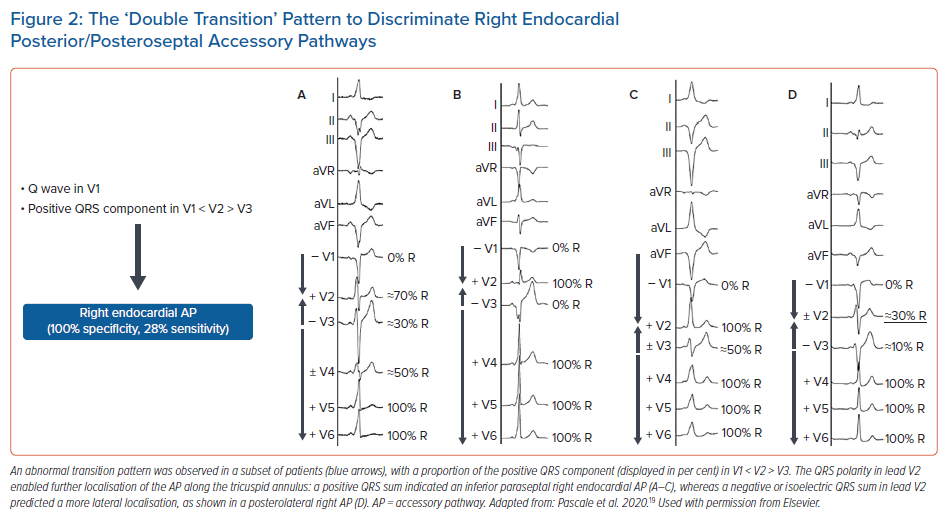
We recently reported a new ECG sign based on the QRS polarity that enables us to specifically identify inferior parasteptal and posterior APs that can be successfully ablated from the right endocardium from those needing another approach.19 Although a progressively increasing R wave proportion across the precordium is normally observed, we observed in a subset of patients an abnormal QRS transition pattern. The ECG sign consisted of the association of either a Q wave, or a predominantly negative wide QRS in V1 (defined as a QRS width >130 ms), with a proportion of the positive precordial QRS component in V2 greater than that in V1 and V3, which produced a ‘double transition’ pattern. This pattern is illustrated in Figure 2. Interestingly, this double transition had been observed by Xie et al., who noted that a little fewer than half of the patients with right inferior paraseptal AP had “a higher R wave in leads V2 and V4 than in V3”.35
In our study population of 273 patients, this pattern was 100% specific for an AP that could be ablated from the right endocardium and could be used to rule out the need for a left-sided approach or an ablation performed within the CS.19 Moreover, the AP localisation could be further refined depending on the QRS polarity in V2. Namely, in the case of a positive QRS, the AP was localised on the right endocardial inferior paraseptal region, whereas in the case of a negative or isoelectric QRS in V2, the AP was localised more laterally on the tricuspid annulus. In that cohort, this double transition pattern helped to characterise the AP localisation of almost one out of seven APs referred for ablation, and almost half of the right endocardial inferior paraseptal APs.
In contrast, regarding the analysis of the QRS polarity in the frontal leads, the analysis of the R/S ratio does not seem to be of meaningful value. An R wave amplitude in lead I exceeding the S wave by ≥1.0 mV has been suggested as a feature that may help discriminate right- from left-sided inferior paraseptal AP.21,32 However, in our experience, most patients with left-sided inferior paraseptal APs indeed have a predominantly positive QRS complexes in lead I (Pascale 2021, unpublished data).
Useful baseline ECG signs to distinguish right endocardial inferior paraseptal APs from left-sided inferior paraseptal APs are summarised in Table 2.
ECG Analysis During Maximal Preexcitation
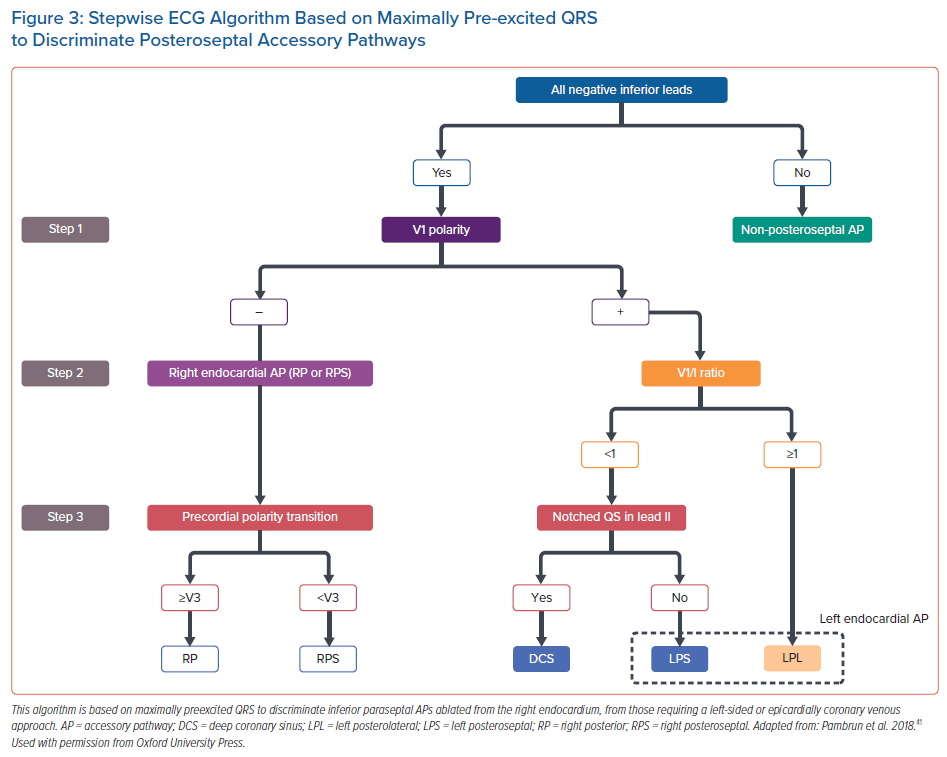
As previously discussed, the analysis of the delta wave and, particularly, the QRS, during baseline ECG may be misleading as a result of the varying degree of fusion with the activation from the His–Purkinje system. Analysis during maximal preexcitation is therefore expected to notably increase the accuracy and reproducibility of the ECG to predict AP localisation. As such, this analysis should be the first diagnostic step in the electrophysiology lab in order to guide the initial mapping strategy. A stepwise algorithm during maximal preexcitation was recently developed by Pambrun et al.41 This four-step algorithm is based on the QRS amplitude and morphology in inferior leads, leads V1, V3 and lead I during rapid atrial pacing. Regarding specifically inferior paraseptal APs and the anticipation of the successful ablation approach, the algorithm can be adapted as illustrated in Figure 3.
First, all inferior paraseptal AP had negative QRS in all three leads. Of note, in the case of isoelectric QRS, the polarity of the QRS was defined by its initial deflection. Second, APs ablated from the right endocardium had a negative QRS in V1, while it was positive for epicardial CS and left endocardial AP. Right inferior paraseptal APs could be distinguished from posterior APs by a positive QRS in V3. Third, epicardial CS APs were identified when the QRS ratio V1/I was <1 (which located the AP left septally), and a notched QS was observed in lead II. The authors raise the hypothesis that this notching may reflect the inhomogeneous ventricular activation related to the various orientations of the sleeve-like CS extensions.
Regarding the algorithm accuracy, the identification was correct in 90% of the patients, as opposed to 63% with the Arruda et al. algorithm. For inferior paraseptal APs, the PPV was 97% and 77% for right and left inferior paraseptal APs, respectively. As expected, the lowest PPV was observed for epicardial CS APs.
Takahashi et al. specifically sought to identify ECG features to discriminate epicardial CS APs from right or left endocardial inferior paraseptal APs during maximal preexcitation.20 They found that a steep positive delta wave in lead aVR had the highest PPV (88%), which increased to 91% when combined with a deep S wave in V6 (R wave ≤ S wave). A positive delta wave in aVR was also reported as a specific finding of epicardial CS APs by Kobza et al.38
In our cohort of APs during maximal preexcitation, we could not reproduce this finding (n=242) (Pascale 2021, unpublished data). Of note, in the study by Takahashi et al. a negative delta wave in V1 was highly specific for right endocardial AP but, unexpectedly, a positive delta wave or a positive QRS were observed in approximately one-third of the patients with right endocardial AP, in contradiction to the above mentioned algorithm. In our cohort, a positive QRS in V1 (as defined in the Pambrun et al. algorithm) was observed in only approximately 10% of maximally preexcited right endocardial inferior paraseptal APs (Pascale 2021, unpublished data).
Nevertheless, one must be aware that whenever a 3D system with precordial patches is used for mapping, the interpretation of V1 or V3 features may be flawed if such leads are recorded from a different position on the patient’s chest.
The 12-Lead ECG Analysis During Orthodromic Reciprocating Tachycardia
Inferior paraseptal bypass tracts generally display negative P waves in all inferior leads and positive P waves in aVR, aVL and V1 during orthodromic reciprocating tachycardia. As can be expected considering the electrical ‘weight’ of the atria, and the difficulties in identifying the P wave morphology with the interference from the T wave, there are no criteria able to discriminate right and left inferior paraseptal APs with reasonable accuracy.42,43
Although present in a minority of patients, the analysis of the ventriculoatrial (VA) interval during functional bundle branch block (BBB) aberrancy may possibly provide some indication to localise the AP on the right or left endocardial side. Lengthening of the VA interval (or of the tachycardia cycle length provided that there are no changes in the atrioventricular interval) by 35 ms or more with the development of BBB indicates the involvement in the tachycardia of an ipsilateral free wall AP.44 Regarding inferior paraseptal pathways, the VA interval may prolong with left BBB albeit to a lesser extent, by 25 ms or less, but it does not vary with right BBB.44,45 Although the absence of VA changes during functional left BBB clearly points towards a successful right-sided approach, VA interval prolongation is often observed in inferior paraseptal APs ablated from the right atrium and CS ostium.18,46 This probably reflects the fact that some APs are actually connected to the posterior superior process of the left ventricle, as discussed previously (Figure 1).
Clinical Perspective
- The localisation of posteroseptal or inferior paraseptal accessory pathways (APs) poses a diagnostic challenge because of the complex anatomy of this region and the fact that a small area may encompass APs that may be approached from the right or left endocardium, or require an ablation performed epicardially inside the coronary sinus.
- There seems to be little evidence to support the fact that a specific ECG pattern enables selective discrimination of APs ablated within the coronary sinus from other APs based on a standard sinus rhythm ECG.
- Considering the limitations in the ECG interpretation, an approach aiming to discriminate subgroups of inferior paraseptal APs ablated from the right endocardium from left-sided APs (endocardial or ablated within the coronary sinus) seems more realistic.
- The baseline ECG signs that seem most useful and relevant to distinguish right endocardial inferior paraseptal APs from left-sided inferior paraseptal APs are based on the finding of a negative delta wave in V1 (depending, however, on how it is defined), the assessment of the R/S ratio in V1, and the finding of a Q wave or predominantly negative QRS in V1 with the ‘double precordial transition’.