Recurrent ventricular tachycardia (VT) in patients who have an implantable cardioverter-defibrillator (ICD) with subsequent shocks is associated with reduced quality of life and an adverse prognosis. Pharmacological treatments are associated with significant side-effects. Catheter ablation has been used to reduce the number of ICD therapies in patients with ischaemic and non-ischaemic cardiomyopathy, and improve VT-free survival. The optimal timing of ablation, however, remains unclear, with a limited number of randomised, multicentre trials suggesting potential benefits of ‘prophylactic’ ablation.
Over the past decade, significant advances in ablation strategies, technology and imaging techniques have been made. A number of large, multicentre, clinical trials are seeking to address some of the unanswered questions in VT ablation using standardised ablation strategies and procedural endpoints. Until we have more data on the impact of VT ablation on mortality, functional status and quality of life, the uptake of ‘prophylactic’ ablation across centres is likely to be limited.
ICDs improve survival in patients with VT. However, patients who receive both appropriate and inappropriate recurrent shocks from their ICDs have an increased risk of death over the medium term, partly due to the progression of heart failure.1 Recurrent ICD shocks can also lead to severe psychological impairment and reduce quality of life, while having little or no effect on the underlying arrhythmogenic substrate.2
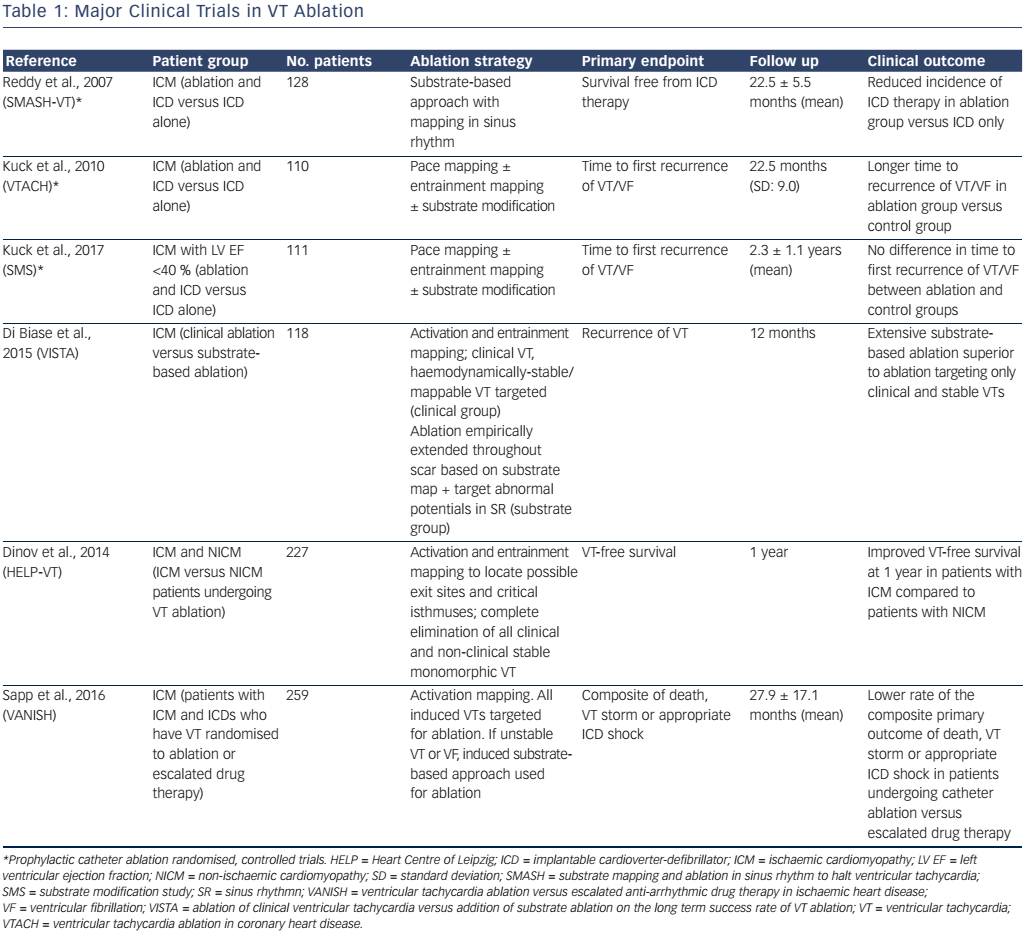
The Substrate Mapping and Ablation in Sinus Rhythm to Halt Ventricular Tachycardia (SMASH-VT) study, first published a decade ago, reported a significant decrease in the number of ICD therapies in patients receiving adjunctive catheter ablation in addition to ICD implantation compared to patients receiving ICD implantation alone.3 Subsequently, the Ventricular Tachycardia Ablation in Coronary Heart Disease (VTACH) study reported an improvement in VT-free survival in patients with ischaemic cardiomyopathy undergoing catheter ablation with ICD implantation versus ICD alone.4 Neither of these studies demonstrated a mortality benefit of catheter ablation. Although the number of catheter ablation procedures for VT has increased over time, there remain unanswered questions about the role of prophylactic catheter ablation at the time of ICD implantation, the optimal timing of ablation, the most effective ablation strategies and which groups of patients derive the greatest benefit.5
Performing randomised controlled trials in VT ablation has proved notoriously difficult (Table 1). The SMASH-VT trial, despite having a 4-year enrolment period across three high-volume centres, recruited 128 patients in total. In order to aid recruitment further, patients who had an ICD for primary prevention, and subsequently received an appropriate ICD therapy, were included following commencement of the trial as an additional qualifying criteria. The VTACH trial had a 3.5-year enrolment period across 16 centres, and recruited 110 patients in total. The VTACH study completed follow up in 2006 and was published in 2010. The pace of progress in interventional electrophysiology has been rapid, with frequent, iterative modifications to ablation techniques, strategies and technologies that are likely to have occurred over this period. Furthermore, optimal ICD programming (high-rate therapy with a 2.5-second delay before initiation of therapy at a heart rate ≥200 BPM, or delayed therapy with a 60-second delay at 170–199 BPM or 12-second delay at 200–249 BPM) can reduce the occurrence of inappropriate therapy, as well as all-cause mortality, compared to conventional programming, which challenges the robustness of an endpoint of either appropriate or inappropriate shocks according to a contemporary definition.6
Although appropriate for the time, neither of the original trials assessing prophylactic VT ablation had what would now be considered optimal ICD programming incorporated into their study protocols.
The SMASH-VT study excluded patients receiving class I or III antiarrhythmic medications, while in the VTACH study, 35 % of patients in each arm received amiodarone, and 75 % of patients received beta-blockers at randomisation. Amiodarone and b-blockers are known to reduce the risk of ICD shocks,7 but interestingly in the Ventricular tachycardia Ablation versus Escalated Anti-arrhythmic Drug Therapy in Ischaemic Heart Disease (VANISH) trial, in patients with ischaemic cardiomyopathy who had VT, escalation in antiarrhythmic drug therapy was associated with a higher composite of death, VT storm or appropriate ICD shock compared to patients who received catheter ablation.8
The VTACH trial reported a cross-over of 22 % from the control group to the catheter ablation group, while seven patients randomised to VT ablation did not receive any ablation due to procedure-related events, lack of appropriate targets, access problems or technical issues.4 A high cross-over rate is difficult to avoid in studies of catheter intervention where blinding of the operator cannot be achieved, and where it would be considered unethical to offer a sham procedure for the management of a potentially life-threatening arrhythmia. There might also be physician bias present when making treatment decisions to enrol patients into a trial that randomises on the basis of having a procedure versus no procedure.
Despite being 10 years on from the first prospective, randomised, controlled trial investigating prophylactic catheter ablation for VT, many centres perform VT ablation often as a last resort late in the disease process, after patients have failed multiple anti-arrhythmic medications, when the procedural risk might in fact be at its highest. A European Heart Rhythm Association Research Network survey of current practice among electrophysiologists in managing VT in patients with ICDs found that most operators performed ablation for recurrent shocks or electrical storm, with prophylactic ablation rarely performed.9 This reluctance to intervene earlier might be due to the continued uncertainty surrounding the impact of VT ablation on disease progression, functional status and mortality in otherwise stable patients. Since these original trials, there have been several advances in imaging techniques, as well as ablation strategies, to guide VT ablation. Both cardiac CT (to assess wall thinning) and cardiac MRI (to assess late gadolinium enhancement/scar) can be used to define the structural substrate for VT. These imaging techniques have been integrated with electro-anatomic mapping to motivate either additional mapping in regions of interest or to determine the need for epicardial access in patients with ischaemic and non-ischaemic cardiomyopathy.10 Using 3D navigator-gated, high-resolution, late gadolinium enhancement MRI to assess myocardial scar, Andreu et al. have suggested that it might be possible to depict channels of slow conduction in border-zone regions of scars with good correlation to electro-anatomic mapping.11 There is ongoing work in this area, and it is conceivable that we might one day be using MRI routinely to define ablation targets and potentially improve procedural success.
There are several techniques to perform VT ablation, including activation and entrainment mapping during VT when the tachycardia is stable or haemodynamically tolerated, pace-mapping when the clinical VT is not inducible or substrate-based strategies. Substrate-based ablation can consist of late potential abolition, elimination of local abnormal ventricular activities, conduction channel ablation and electric isolation of low voltage areas and scar homogenisation.12 Differences in mapping techniques and ablation strategies used make comparisons between studies difficult to interpret. The SMASH-VT study employed a combination of pace mapping and/or entrainment mapping to identify regions of arrhythmogenic tissue within an area of infarct and target for ablation, while the VTACH trial also allowed for substrate modification (defined as the absence of all channels within an area of interest) in patients with non-inducible VT.3,4 The SMASH-VT study performed ablation of late or fractionated potentials in a small group of patients with severe ventricular dysfunction. Advanced imaging techniques to define myocardial substrate in 3D were not available for either study.
There has been recent interest in substrate-based ablation strategies to guide VT ablation over ablation limited to clinical and mappable VTs. The multicentre Ablation of Clinical Ventricular Tachycardia versus Addition of Substrate Ablation on the Long-term Success Rate of VT Ablation (VISTA) trial randomised patients with ischaemic cardiomyopathy to either substrate-based ablation where all ‘abnormal’ electrograms within scars were targeted, or limited ablation of clinical VT guided by pace mapping, activation mapping and entrainment mapping. There were 60 patients included in the ‘clinical ablation’ arm and 58 patients in the ‘substrateguided’ arm, and while there was no difference in mortality between the two groups, there was an improvement in the cumulative recurrence probability of VT using the substrate-based approach.13
In this context, the recently-published substrate modification study (SMS) adds an important perspective. A total of 111 patients with ischaemic cardiomyopathy and left ventricular ejection fraction (LV EF) <40 % were randomised to either catheter ablation prior to ICD implantation or ICD implantation alone. Over a follow up of 2.3±1.1 years, there was no difference in the primary endpoint–time to first recurrence of VT/VF. However, there was a >50 % reduction in the total number of ICD interventions during follow up in the catheter ablation arm.14 There were significant differences in the design of the SMS compared to the SMAST-VT and VTACH trials, which could account for some of the findings. In the SMS, 27 % of patients received amiodarone, while these patients were excluded from SMASH-VT. There were differences in ICD programming between studies, which might have accounted for differences in recurrence rates between trials. The procedural endpoint in SMS was non-inducibility of VT with or without additional substrate modification, while substrate modification was a predefined endpoint in VTACH when VT was non-inducible. Therefore, it is possible that some patients with noninducible VT might have had more extensive ablation in the VTACH trial compared to the SMS. Enrolment into the SMS took 7 years to complete, echoing the problems encountered with recruitment in previous VT ablation clinical trials.
Previous work has shown significant differences in the procedural success of catheter ablation depending on the patient group studied. The Heart Centre Leipzig (HELP)-VT study demonstrated significantly worse VT-free survival in patients with non-ischaemic cardiomyopathy versus patients with ischaemic cardiomyopathy (40.5 % versus 57.0 % at 1-year follow up).15 The underlying substrate might be different in non-ischaemic cardiomyopathy, where epicardial ablation is more frequently required. Subgroup analysis from the VTACH trial suggested that patients with an LV EF <30 % derived no benefit from ablation in terms of VT-free survival, whereas those with EF >30 % derived a greater benefit. 4 This observation might also account for the failure to reach the primary endpoint in the SMS, where all patients enrolled had a LV EF <40 %, leading to a possible underestimation of the potential benefit of VT ablation. These studies provide important insights into which patients should be offered catheter ablation.
One of the limitations of clinical trials in VT is the lack of a standardised definition of acute procedural success with studies defining noninducibility of ‘clinical VTs’ as procedural success, while others define complete non-inducibility, including all late potential abolition, as procedural success. It remains unclear whether additional ablation to achieve complete scar homogenisation is required for long-term success. Without uniform procedural endpoints, it is difficult to compare the quality of ablation between different clinical trials.
VT ablation can be a challenging procedure and is associated with a significant risk of complications, including cardiac tamponade, stroke, vascular injury, thromboembolism, perforation as well as procedurerelated death. While the risk of complications in idiopathic VT ablation might be comparable to other low-risk ablation procedures, the risks of VT ablation in structural heart disease is considerably higher. A recent retrospective analysis from a large, multicentre registry of >2,000 patients undergoing VT ablation in structural heart disease found an early mortality rate of 5 % within 30 days of the procedure, while the cumulative overall mortality at 1 year was 13 %.16 The procedure itself can also be time consuming, which could have potential implications on resource allocation if more VT ablations were to be performed on a prophylactic basis. These issues could limit the applicability of prophylactic VT ablation to high-volume tertiary centres with more expertise and resources to avoid and/or deal with complications.
There is limited evidence to suggest that clinicians are considering catheter ablation for VT late in the disease process in the realworld setting. The Pilot Study of Catheter Ablation for Ventricular Tachycardia in Patients with an Implantable Cardioverter Defibrillator (CALYPSO) pilot trial suggested that most patients had already failed anti-arrhythmic medications prior to being considered for VT ablation, and that these patients subsequently had high rates of VT recurrence and death.17 ‘Prophylactic’ catheter ablation, either prior to or at the time of ICD implantation, might therefore allow modification of arrhythmia substrate early in the disease, and potentially allow the full benefits of ablation to be realised. The three main randomised clinical trials assessing prophylactic catheter ablation for VT described above have given some important insights into which patients might derive the greatest benefit from ablation, and how differences in ablation strategy could affect clinical outcome, but more evidence is required. Unanswered questions include:
- Should patients with structural heart disease undergoing ICD implantation be considered for prophylactic substrate ablation in the absence of VT?
- What is the appropriate ablation strategy and endpoint in an individual patient?
- At what point in a patient’s history is a catheter intervention for VT appropriate?
There are a number of ongoing clinical trials which could shed some light on some of these questions, as well as provide important data that could help us understand the role of VT ablation. The preventive ablation of ventricular tachycardia in patients With myocardial infarction (BERLIN) VT trial is a prospective, randomised, multicentre study that aims to assess the impact of prophylactic VT ablation prior to ICD implantation compared to ICD implantation and best medical care until a third appropriate shock and catheter ablation thereafter. The study will aim to recruit over 200 patients and assess the impact of prophylactic VT ablation on all-cause mortality and unplanned hospital admissions for congestive heart failure or VT/VF as the primary endpoint. The trial will aim to complete recruitment at the end of 2018 and should provide insights into the role of VT ablation in reducing mortality, hospital admissions as well as assessing its impact on functional status and quality of life (secondary endpoints).
The substrate targeted ablation using the flexAbility™ ablation catheter system for the reduction of ventricular tachycardia (STAR)- VT trial, which has recently completed its enrolment phase, is a large, multicentre, randomised, controlled trial investigating the impact of scar-based VT ablation compared to routine drug therapy on freedom from ICD shocks and cardiovascular-related hospitalisations. The study will recruit patients with both ischaemic and non-ischaemic cardiomyopathy, while patients in both study arms will receive ICD/ cardiac resynchronisation therapy-defibrillators (CRT-Ds) and routine drug therapies. This study will help us address the question of whether VT ablation results in fewer appropriate ICD therapies and VT recurrences compared to anti-arrhythmic drugs using modern-day technologies and a standardised substrate-based ablation strategy.
The PARTITA trial, investigating whether timing of VT ablation affects prognosis in patients with an implantable cardioverter-defibrillator, is an ongoing multicentre, randomised, controlled trial that aims to recruit 590 patients by late 2018. This study will randomise patients to receive catheter ablation either immediately after an appropriate ICD shock, or to delay ablation until an arrhythmic storm occurs. The findings of this trial will be crucial in helping to determine what the optimal timing of VT ablation should be.
In conclusion, although there is some evidence that prophylactic VT ablation might be of benefit in some groups of patients, but this evidence cannot be described as compelling. More data are needed from trials utilising contemporary advances in ablation technologies and cardiac imaging with standardised ablation strategies, procedural endpoints and follow-up protocols. In this regard, the ongoing trials described here might provide important and exciting data to support the need for prophylactic VT ablation, but we are not there yet.