Radiation-associated cardiovascular disease (RACD), sometimes referred to as radiation-induced cardiovascular disease, is an increasingly recognised clinical entity.1 It is a significant adverse effect of radiation therapy for common malignancies that involve the chest, and includes lymphomas, lung, mediastinal and breast cancers.2 The acute manifestations of RACD include inflammatory reactions, such as pericarditis and/or myocarditis, while chronically, fibrosis leading to cardiomyopathy, valvular dysfunction, coronary artery disease and constrictive pericarditis develop years later in life and have been well-studied.3 However, abnormalities of the cardiac electrical conduction system and arrhythmias secondary to radiation are less characterised. With the increasing incidence of AF in an ageing population, which is associated with significant cardiovascular complications, it is vital to understand the risks of radiation therapy. This is particularly important, as cardiovascular disease in cancer survivors is the second leading cause of morbidity and mortality.4 In this focused review, we present an overview of RACD as one of the possible aetiologies of arrhythmia development after radiation therapy, consider the pathophysiological basis and diagnosis of radiation-associated arrhythmias (RAA), and discuss its possible risk factors, screening guidelines and treatments.
Online searches were conducted on the MEDLINE/PubMed database between January 1990 and December 2021. An initial review of original studies identified by the search engine was performed and all relevant studies were included in our review. Broad search terms were used including the following keywords: ‘radiation’, ‘radiation injuries/radiation exposure/radiation effects’, ‘chemoradiation’, ‘arrhythmia’, ‘radiation-associated cardiac disease’, ‘cardiac device’, ‘defibrillator’ and ‘pacemaker’. We mainly focused on English language studies published in the past decade. An attempt was made to cite representative literature, so not all references are listed. Knowledge from our clinical experience was used to supplement some of the gaps in the literature and will be clearly listed in text when mentioned. Cardiac events (CE) discussed in the studies reviewed included acute ischaemic events, heart failure exacerbations, arrhythmias needing cardioversion, emergency department visits, hospitalisation or device placement and valve replacements.5
History and Epidemiology
Radiation-associated conduction disturbance was first described in 1981 in a case report of a 20-year-old patient with Hodgkin’s lymphoma who was treated with mantle radiotherapy and then developed predominant junctional rhythm with intermittent complete heart block. The patient died from meningitis a few months later, and autopsy examination of his heart demonstrated marked fibrosis of his sinoatrial node, atrioventricular node, and proximal parts of his right and left bundles.6 After this, larger case series were described. Borkenhagen et al. performed a retrospective review of 76 patients treated with radiotherapy for lung cancer, noting 22 CE, with five (6.5% of total subjects) being new atrial arrhythmias. Further analysis demonstrated target areas receiving >45 Gy dose resulted in an increased incidence of major adverse cardiovascular events (defined as a composite of total death, MI, stroke, heart failure-related hospitalisations and revascularisation; HR 1.50; p=0.027), and the occurrence of RACD was found to be a significant predictor of overall mortality (HR 12.7; p<0.001).7
In another analysis, Wang et al. analysed 112 patients who underwent radiotherapy for stage III non-small cell lung cancer over 8.8 years, and found that 12 (10.7%) experienced new-onset arrhythmias, including AF (66.7%), atrial flutter (16.7%), complete heart block (8.3%) and sick sinus syndrome (8.3%). These findings were seen at a median of 23 months post-radiation, requiring two patients to undergo temporary or permanent pacing, one cardioversion and one ablation. The authors found that there was a non-significant trend towards development of arrhythmias with increasing radiation exposure.8
The findings by Wang et al. are similar to those of a study by Reshko et al., where 74 patients treated with radiotherapy for early-stage lung cancer presented with 18 CE, of which eight were new-onset arrhythmias, with findings at 19 and 26 months, respectively.5 However, the actual incidence of arrhythmias may be underestimated, given the relatively short follow-up with an average of 2.2 years.
In a 5-year retrospective review of 80 patients who underwent chemoradiation for submucosal oesophageal cancer by Hayashi et al., 13 patients were found to have CE, including one subject with new-onset AF, two with sudden cardiac death, three with pericardial effusions and seven with ischaemic heart disease. Further analysis showed a dose–response relationship between levels of radiation and the incidence rate of CE. Again, it appears the proximity of targeted tissue, in this case, the oesophagus to the atrium, may be a risk factor for CE following radiation; in fact, the authors suggest surveillance of these patients for several years following therapy.9
Pathophysiology
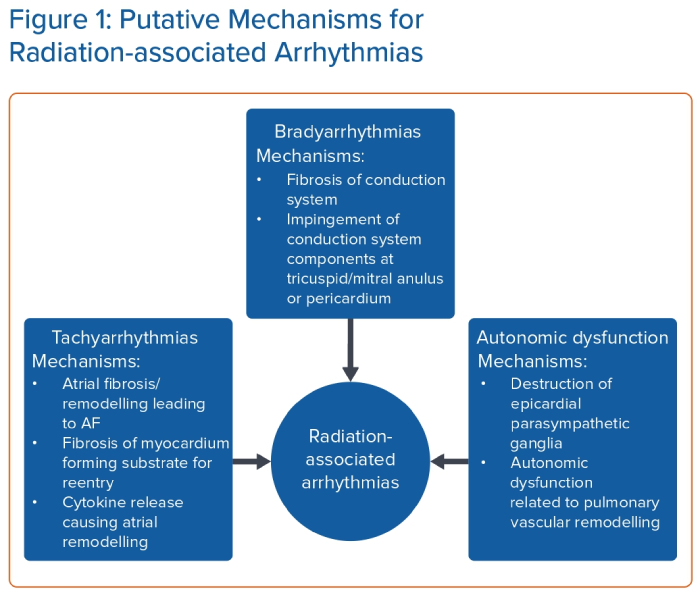
The association between radiation therapy and the occurrence of arrhythmias has been well observed in clinical studies, with evidence pointing to an increased risk of bradyarrhythmias, tachyarrhythmias and autonomic dysfunction in patients who have undergone radiation treatment. However, the underlying pathophysiology of this disease process is still not fully understood (Figure 1), and there are several gaps in our knowledge. The exact mechanisms by which radiation induces different types of arrhythmias, characterised by variable onset and severity, remain elusive and require further investigation.
The role of radiotherapy in the treatment of malignancies is to perturb cellular division through alteration of DNA replication, and alter lipid and protein signalling pathways. Indirect ionising radiation causes free radical formation, while direct ionising radiation breaks single and double-strand DNA directly. These fundamental mechanisms of radiation are dose-dependent, and further increases in the pre-specified radiation dose are limited by adverse effects on healthy tissue.10
Bradyarrhythmias
Direct radiation injury and subsequent fibrosis of the conduction system is the most likely aetiology of bradyarrhythmia associated with radiation. Other possible mechanisms include secondary causes, such as calcification of the tricuspid/mitral annulus or the pericardium, which typically occurs several years following radiation exposure.11 Calcifications at the tricuspid and mitral annulus could cause compression of the atrioventricular node or the His bundle due to its close proximity to these structures.12 Constrictive pericarditis or calcification of the pericardium may also compress or irritate the sinus node, a structure that is exposed to the epicardium of the heart.13
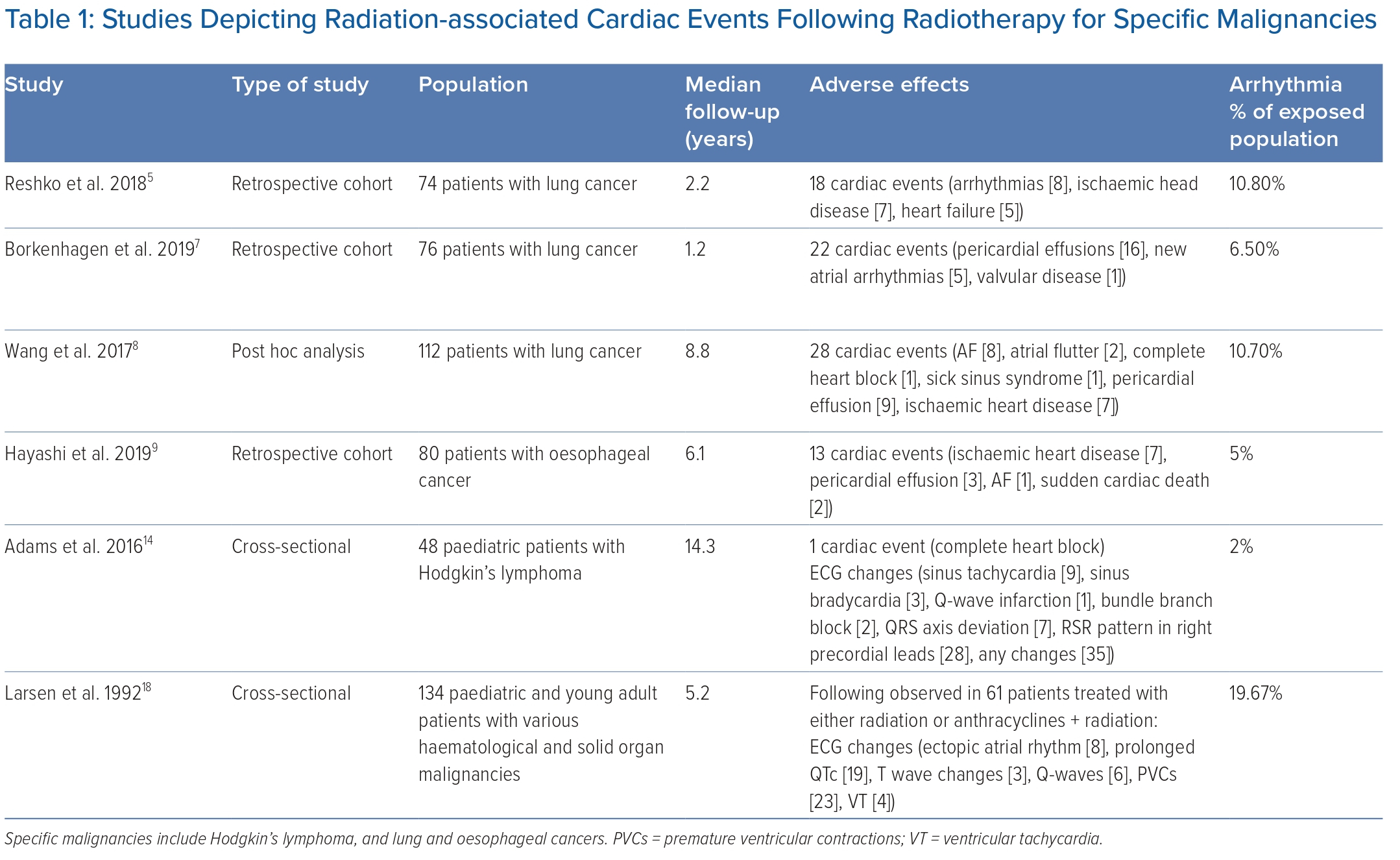
Compared with tachyarrhythmias or autonomic dysfunction, a robust link has been established between radiation exposure and the development of fibrosis in the conduction system leading to bradyarrhythmias. This is supported by compelling evidence, including the presence of right bundle branch block and ‘rSr’ pattern (possibly incomplete right bundle branch block) in patients who have undergone radiation therapy. For example, Adams et al. analysed 48 patients treated with radiotherapy for Hodgkin’s lymphoma at a median of 15.5 years, and ECG changes were present in 75% of cases (Table 1). One patient developed complete heart block and the majority of ECG changes exhibited an RSR’ pattern in the right precordial leads, which may be related to the anatomical location of the right bundle system and its propensity for fibrosis.14
The risk of developing bradyarrhythmias after radiation exposure is further highlighted by comparing the incidence of permanent pacemaker implantation in this population versus the population in general. A recent study published by Greenspon et al. estimated the incidence of implantation of permanent pacemakers for all indications to be 61.6/100,000 patients within the general population.15 The incidence of permanent pacemaker implantation appears to be higher in patients who have received radiation therapy compared with the general population, although no direct head-to-head comparisons have been previously conducted.3,5,8,9,16 The incidence of complete heart block is also considerably higher in this population when compared with the general population (0.02–0.04%).15 All these findings suggest that radiation-induced fibrosis disrupts the normal conduction pathways in the heart, leading to irreversible damage and requiring permanent pacemakers in some cases.
Tachyarrhythmias
The relationship between RAA and tachyarrhythmias is not as clear. While the rate of tachyarrhythmia development after radiation exposure, such as AF, is much higher than the general population based on our literature review (Table 1), the prevalence of AF increases with age, up to 18% in those aged ≥85 years, and may not be any different when adjusted for by age.9,17 There is also a significantly lower incidence of atrial arrhythmias in the paediatric population, which traditionally has very low incidence of AF.14,18
However, fibrosis of the atrium, which is possible following oesophageal radiation treatment given their close proximity to one another, is a well-known substrate for the development of AF. Several studies have previously demonstrated the causal and reciprocating relationship between AF and atrial fibrosis.19 In addition, atrial fibrosis was also associated with poor AF ablation outcome/recurrence and left atrial appendage thrombosis.19,20 Limited research has been conducted on the association between radiotherapy and atrial fibrosis, with only one study demonstrating a dose-dependent relationship between radiation dose and left atrial scar/fibrosis in seven patients receiving external radiation beam therapy.21 Fibrosis is also the essential substrate for the evolution of re-entrant arrhythmias, such as atypical flutter.22 Another plausible aetiology may be that direct injury to the lung or oesophagus from radiation causes the release of inflammatory cytokines, leading to atrial remodelling and predisposition to arrhythmia development.23,24
Indeed, the incidence of atrial tachyarrhythmias is high in lung cancer and oesophageal cancer patients who undergo radiotherapy, which appears to suggest anatomical proximity to the heart during radiation plays a secondary role in the development of radiation-induced atrial tachyarrhythmias.
Autonomic Dysfunction
Although technically not an arrhythmia, autonomic dysfunction is a phenomenon observed years after radiation treatment and can mimic symptoms of cardiac arrhythmias.1,25 Groarke et al. described abnormally high sinus heart rates and abnormal heart rates following exercise in patients.25 In that study, 263 Hodgkin’s lymphoma survivors were referred to exercise testing at a median 19 years after initial radiation therapy. They found that prior radiation exposure was associated with abnormal resting heart rate and heart rate recovery after adjusting for age, sex and cardiovascular risk factors.25 The precise aetiology is unclear, but there are two plausible explanations for this observation. Superficial vagal ganglia are situated on the epicardial surface of the right atrium and help mediate parasympathetic responses, such as bradycardia.26 Destruction of these ganglia by radiation could cause unopposed sympathetic activity, leading to tachycardia. In fact, intentional destruction of these parasympathetic epicardial ganglia by cardiac ablation has been used to treat symptomatic sinus bradycardia, elevating the heart rate by an average of 10–20 BPM in one case series.27 Another possible explanation for the development of autonomic dysfunction after radiation treatment is pulmonary vascular remodelling leading to pulmonary arterial hypertension. Lung radiation has been shown to cause vascular remodelling which resembles that seen in pulmonary arterial hypertension.28 Patients with pulmonary hypertension, specifically those with pulmonary arterial hypertension, have been shown to demonstrate evidence of autonomic dysfunction during autonomic function testing.29 Although it is unclear if the patients studied by Groarke et al. had pulmonary hypertension, this is one of many important knowledge gaps in the area of RAA that needs to be addressed.
Special Populations
Patients with cardiovascular implantable electronic devices (CIEDs) who are undergoing radiotherapy may be prone to adverse effects from device malfunction secondary to electromagnetic interference, secondary radiation via scattering and direct damage to the device’s random-access memory. Some observed malfunctions include programming resets and signal interference causing alterations to sensing and pacing thresholds.30 A retrospective analysis of radiotherapy-induced CIED malfunction conducted by Grant et al. in 2015 identified 215 patients with CIEDs who underwent 249 courses of photon- and electron-based radiation therapy between 2005 and 2014. Analysis showed that neutron-producing radiation treatments (typically associated with high-energy photon beam therapy) caused the majority of device malfunctions out of a total prevalence of approximately 7% of treatment courses; approximately 16% of these went on to develop clinical symptoms related to the CIED malfunction.30 Although these findings have been reported to be limited to high-dose therapy, current guidelines recommend a complete CIED evaluation be performed before radiotherapy (class 1), weekly evaluations for patients receiving neutron-producing radiotherapy (class 1) and no relocation of the device when the maximum cumulative incident dose is to be <5 Gy (class 3).31
Prevention and Screening
With the advent of more precise and efficient radiation therapy techniques, it is hoped that RACD incidence will decrease.32 However, with an ageing population more prone to cancers and the increased detection of these diseases, the prevalence of RAA will likely increase.33 Currently, screening guidelines for RAA are limited to patients with pre-existing CIED, but none are established for the remaining population. Different Gy levels are found in the literature as an initial inclusion criterion for patients most likely to benefit from screening. A review of current literature suggests that the population group most likely to benefit from these screenings are those patients who are exposed to a cumulative radiation dose in the range of 30–40 Gy.2
The American Society of Echocardiography proposed an expert consensus in 2013 for screening for RACD, recommending screening with a transthoracic echocardiogram, cardiac MRI or coronary CT angiography in patients who have received >35 Gy of radiation, either 5 years after completion of therapy or after ages 30–35 years, whichever occurs later.34 Although the screening modalities in this recommendation do not apply specifically to arrhythmias, its inclusion criteria for screening can be adopted to screen patients for RAA. Screening for RAA with the above-mentioned modalities should be considered in patients with the following: onset of cardiac symptoms several years after initial treatment and a total radiation exposure >30 Gy. Other factors to consider while evaluating the need to screen for RAA include age <50 years at the time of presentation with cardiac symptoms, anterior or left chest irradiation location and initial tumour burden around the heart.35
Most experts recommend screening for RACD 5 years after radiation exposure.1,34 The simple and non-invasive ECG is an ideal test to screen for conduction abnormalities and potential bradyarrhythmias due to the well-established link between RAA and bradyarrhythmias. However, given the potentially serious nature of tachyarrhythmias, screening for tachyarrhythmias should also be considered. Ambulatory monitoring is perhaps the best screening method, but it is unclear what the ideal duration of monitoring is, especially in an asymptomatic patient. In a patient previously treated with radiation, palpitations or symptoms suggestive of arrhythmia should definitely be evaluated with ambulatory monitoring. In summary, a screening ECG provides quick and valuable information for early detection and management of arrhythmias in patients who have undergone radiation therapy, particularly bradyarrhythmias.
Diagnosis and Treatment
There are currently no established guidelines for the diagnosis of RAA. However, radiation-associated conduction abnormalities usually occur years after initial exposure. Previously, a review of 19 patients sought to define atrioventricular block due to radiation using five criteria, including a total radiation dose >40 Gy; an onset of disease ≥10 years after therapy; an abnormal interval ECG tracing; prior pericardial involvement; and associated cardiac or mediastinal lesions.36 While it is unknown if an arrhythmia that is associated with radiation confers greater morbidity or mortality than the same arrhythmia that has arisen de novo, treatment of these arrhythmias should be no different between the two. However, while treatment of RAA is identical to the same arrhythmia in general, the astute clinician needs to maintain a high index of suspicion that an arrhythmia is afoot when a history of radiation exposure is present, and there should be a lower threshold to perform screening tests, such as ECG or ambulatory monitoring, in these patients.1,36
Other comorbidities are likely to imperil an already vulnerable population to radiation-associated arrhythmia development. However, Hayashi et al. found neither smoking, hypertension, diabetes and hyperlipidaemia nor a history of cardiac disease were prognostic of CE.9 Moreover, usage of chemotherapy causing chemical cardiotoxicity in addition to RACD may cause an increase in CE, for which further retrospective and prospective studies are required.
Conclusion
Radiation can have many adverse cardiovascular effects, including the development of bradyarrhythmias, tachyarrhythmias and autonomic dysfunction. The onset of arrhythmias has been reported anywhere from 5 to 38 years following therapy, with incidence ranging between 2% and 19% of cases. The current literature available on radiation-associated arrhythmias is limited to a handful of single-centre retrospective reviews, cross-sectional studies and post-hoc analyses, which, given the growing clinical importance of this topic, is modest at best. In this focused review, it appears that radiation exposure in the anatomic location of radiation therapy and radiation doses used may play a role in increased incidence of arrhythmia development in the years following treatment, although confounders need further assessment.
A common limitation across numerous studies cited in this review is the retrospective nature of current evidence available. Without baseline risk factors and comorbidities clearly elucidated, there is potential for selection bias. Further prospective research into this topic is needed, with longitudinal follow-up and cardiac testing. This will allow for better characterisation of the relationship between radiation therapy, radiation dosing and development of arrhythmias. The above findings of this review, combined with improved cancer survival and an ageing population, should prompt further studies to expand the limited extent of the current literature on the topic, and explore the relationships between radiation exposure/doses and the development of future arrhythmias, screening and prevention strategies, and nuances to management. 
Clinical Perspective
- Radiation-associated arrhythmias are an important subset of adverse effects arising from radiation exposure for treating malignancies.
- The rate of arrhythmia development based on this review varies from 2% to 11%, with atrial tachyarrhythmias being the most prevalent arrhythmias.
- Screening for radiation-associated arrhythmias should be considered in patients with cardiac symptoms and prior total radiation exposure >30 Gy.
- Current management of radiation-associated arrhythmias is the same as for non-radiation-related arrhythmias, and includes pharmacological, device-based and catheter ablation treatments.