Transseptal catheterisation (TSC) involves the use of specialised equipment to gain access to the left atrium (LA) after crossing the interatrial septum (IAS) from the right atrium (RA) during cardiac interventional, electrophysiological and surgical procedures. The long journey to where we are today in regards to the tools and techniques used to perform this procedure started in the 1950s. Back then, in order to obtain access to the left-sided cardiac chambers, several relatively cumbersome techniques were under investigation. These included the suprasternal approach, the posterior transthoracic approach, the transbronchial approach and the direct left ventricular puncture via the apical or the subxiphoid approaches.1 In 1959, John Ross at the National Heart Institute described a novel method to access the LA via a transseptal puncture.2 This technique was further refined by Edwin Brockenbrough in conjunction with Eugene Braunwald with the addition of a smaller 21 G needle and a tapered catheter, enabling percutaneous access to the right femoral vein and obviating the need for a saphenous vein cut down.3 This was followed by a period of stagnation with the advent of retrograde aortic access performed via transcutaneous approach, which was considerably less complex and enabled access to the left side of the heart. With time, as the need to approach the left heart for various valvular interventions arose, interest in obtaining transseptal access rekindled. In 1983, Charles Mullins described excellent results with the new Transseptal Introducer Set, which consisted of a curved sheath with a rigid dilator that was designed to fit the Brockenbrough needle inside it.4 Since then, the equipment has evolved considerably to improve the success rates of TSC and to further reduce procedural complications. The use of needles and guidewires capable of delivering radiofrequency (RF) energy from their tips and providing a safe rail to support the delivery of the introducer sheaths into the LA has added to the safety of TSC and has simplified this procedure in many ways.5–7 Several commercially available transseptal systems are summarised in Table 1. The TSC procedure has been rendered even simpler with the use of direct imaging with transoesophageal echocardiography (TOE) and intracardiac echocardiography (ICE) to the extent that many centres perform TSC without the use of fluoroscopy, thus reducing patient and operator exposure to ionising radiation.8,9 The use of 3D electroanatomic mapping systems with the ability to incorporate ICE images, such as the Carto 3 system (Biosense Webster), which can also visualise sheaths, improves procedural efficiency and decreases radiation exposure by minimising fluoroscopy time.
In this new era of catheter-based procedures for various arrhythmias and valvular disorders, the skill set for the safe performance of TSC is essential. Despite the fact that this procedure has become ubiquitous, there are still scenarios in which TSC is challenging and even considered contraindicated. This review will focus on several such scenarios and the unique solutions to circumvent them.
General Anatomical Considerations
A correct understanding of the anatomy of the atrial septum is crucial to safely perform TSC. When the heart is in its attitudinal position, the RA is positioned rightward and anterior while the LA is a leftward and posterior structure: as such, the plane of the IAS is slanted from left anterior to right posterior. Fluoroscopically, the IAS is almost perpendicular to the plane of the screen in the left anterior oblique projection and faces the plane of the screen in the right anterior oblique projection. The overall area of overlap between the RA and LA (true IAS) is only at the level of the fossa ovalis and the muscular rim or limbus. Although the rim is part of the true septum, it should not be targeted for TSC in order to avoid complications, such as intramural septal hematoma, particularly in fully anticoagulated patients. The fossa ovalis, a fibromembranous structure of 0.5–1.5 mm in average diameter, is the only structure that should be targeted for TSC. Of note, the muscular rim around the fossa ovalis is not always a clearly distinct structure, and occasionally a gradual thinning can be encountered without a clear muscular rim. In these instances, direct intraprocedural imaging of the IAS with either TOE or ICE is important, given that operators may not be able to rely on the tactile or fluoroscopic ‘jump’ when withdrawing the transseptal sheath and needle from the superior vena cava to the fossa ovalis. The following section will review several challenging TSC scenarios, emphasising the benefit of intraprocedural imaging (particularly with ICE) in these cases.
Cases of Challenging Transseptal Catheterisation
Sclerotic Interatrial Septum
A frequent challenge for transseptal access is a sclerotic IAS, which may be the result of prior TSC and cardiac surgery but which has also been reported in patients without a history of prior invasive procedures (e.g. in patients with myocardial calcinosis in the setting of chronic renal failure).10,11 At times, the initial ICE images can be deceiving and the only clue lies in the fact that the IAS is more echogenic than usual. This poses a unique challenge because the operator is forced to apply more than usual forward pressure, which may be associated with increased risk of perforation and pericardial effusion leading to tamponade. This is especially true when TSC is used to perform catheter ablation for AF.12 Several approaches can be used to perform successful TSC in such cases. First, there is often variability in the thickness of the IAS, which is visible only when a detailed examination is conducted with direct imaging of the IAS. Using ICE, areas of septal thinning can be used to the operator’s advantage and targeted for TSC. Large sheaths and sheaths with a step-up between the dilator and the sheath may be difficult to manoeuvre across the sclerotic IAS. In such cases, crossing the septum with a smaller calibre sheath first and then exchanging to a steerable sheath using a stiff 0.035 inch guidewire is helpful. The use of TSC systems that have a needle capable of delivering RF energy, such as the NRG transseptal needle, can be helpful to access the LA efficiently.13 Once access to the LA is achieved, another technique that can be helpful is advancing the steerable sheath over the dilator with constant clockwise or counterclockwise rotation over a stiff guidewire, or simply advancing a specialised J-tipped guidewire designed for safe IAS perforation, such as SafeSept (Pressure Products), to the pulmonary veins and advancing the BRK needle over the wire to achieve a stable rail. Several newer tools, such as the VersaCross Access Solution (Bayless) and AcQCross Transseptal Access System (Acutus Medical), that incorporate RF energy application to assist with TSC, can simplify the procedure and obviate the need for repetitive sheath exchanges. If these techniques fail, bailout balloon atrial septoplasty can be used to facilitate the passage of the sheath within the LA.14
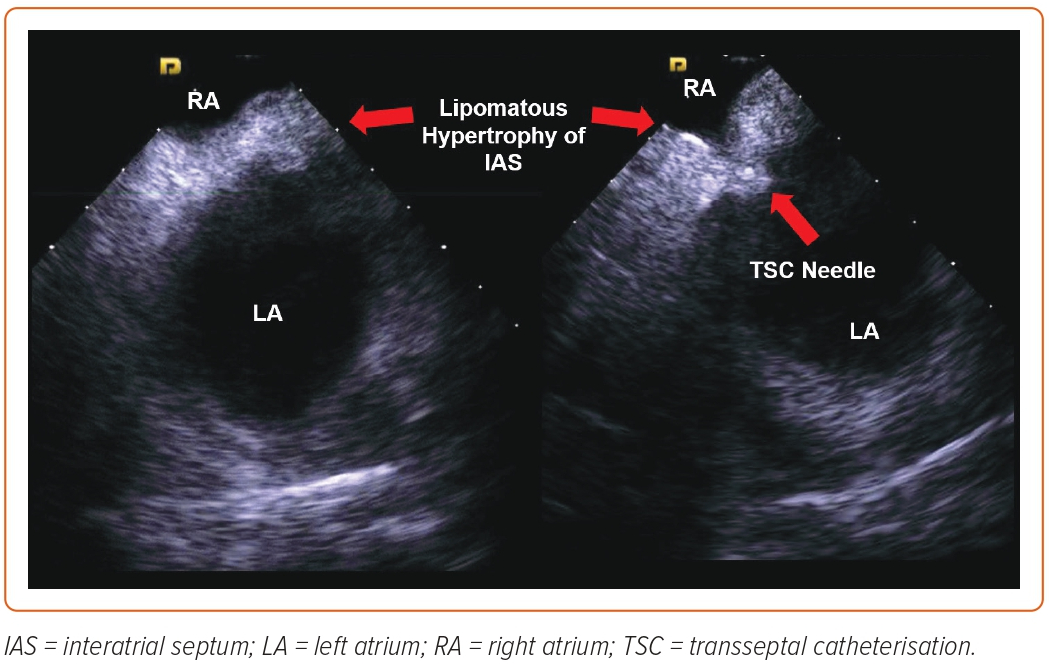
Lipomatous Hypertrophy of Interatrial Septum
This abnormality is characterised by lipomatous infiltration of the IAS involving the limbus of the fossa ovalis and is found in around 2.2% of patients. It is associated with obesity and atrial arrhythmias and its incidence increases with older age.15 Fortunately, the central portion of the fossa ovalis is usually spared: the lipomatous infiltration usually creates a funnel-shaped IAS as seen on 3D echocardiography, and a dumbbell-shaped IAS as seen on ICE, which can be instrumental in guiding the TSC apparatus to the thinnest portion of the IAS, where the abovementioned techniques are invariably successful (Figure 1). Rarely, septoplasty is needed if the thicker portion of the IAS is crossed.14 Care should be taken to avoid entering the lipomatous parts of the IAS because this increases the risk for intramural hematomas.
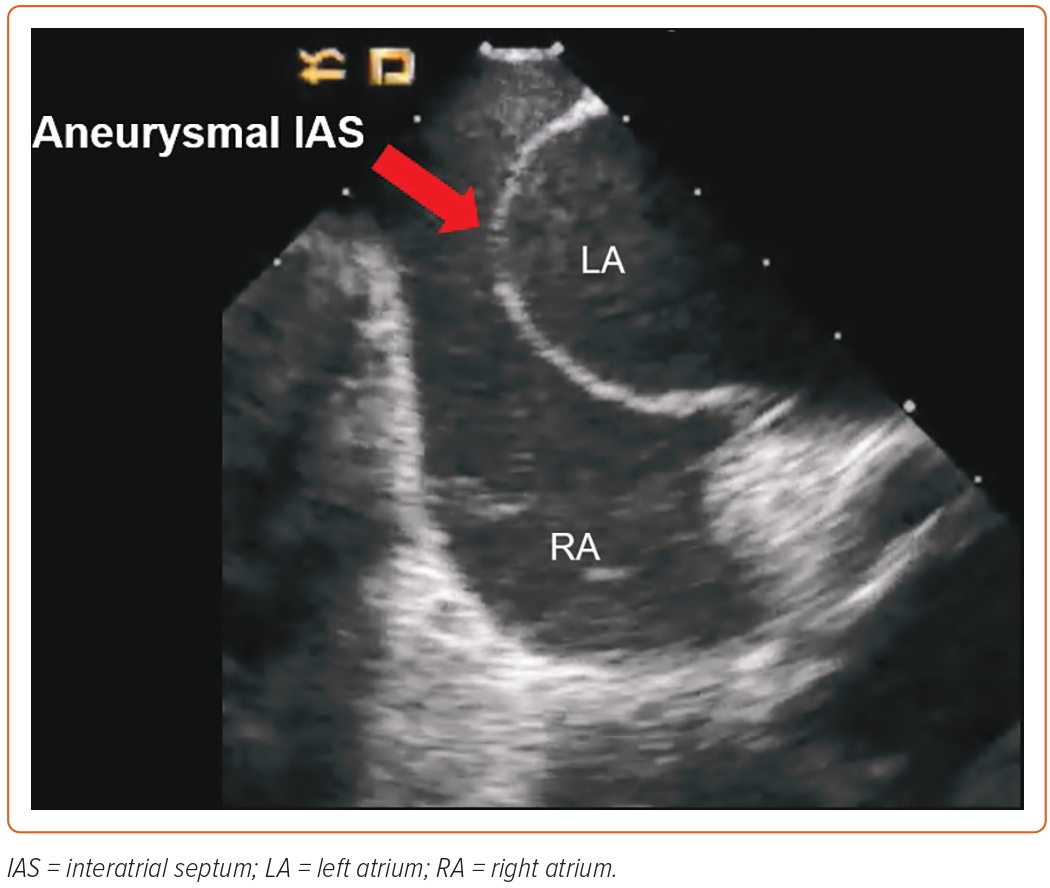
Aneurysmal Interatrial Septum
Another challenging scenario is the presence of a hypermobile or aneurysmal IAS, defined as greater than or equal to 10 mm excursion of the IAS with respiration.16 This is difficult to visualise on fluoroscopy but is suggested by a more than usual amount of leftward displacement of the tip of the dilator in left anterior oblique projection as it drops to the fossa ovalis. Direct visualisation of the IAS with ICE is of paramount importance in these cases, given that the forward pressure applied by the dilator can place the TSC apparatus close to the lateral wall of the LA and risk perforation.17 This does not allow much room in the LA once the needle crosses the IAS and several techniques can be used to facilitate safe TSC. One manoeuvre is the use of ICE to point the needle in a direction that maximises the space in the LA. In some cases, this is not feasible and exchanging the needle for a stiff guidewire right after piercing the IAS creates a safe rail over which the guidewire and sheath can be advanced in the LA. Depending on the atrial anatomy and the site of TSC, it is easier to advance the TSC apparatus over the guidewire in the right superior pulmonary vein because it provides a straighter path when compared with the left pulmonary veins. The use of TSC needles or wires capable of delivering RF energy also enables safe puncture of the IAS without the need for excessive forward pressure. In the case of wires capable of delivering RF energy, for example, the VersaCross Access Solution (Bayless) and the AcQCross Transseptal Access System (Acutus Medical, an entire step can be skipped and the TSC apparatus can be advanced over the same wire into the LA, knowing that trauma to the lateral or posterior wall of the LA is less likely (Figure 2). Another important fact to keep in mind in such cases is that excessive curvature of the TSC system can make it difficult to balance it on the aneurysmal septum and the TSC system can easily move anteriorly, posteriorly and inferiorly by the coronary sinus ostium, increasing the risk for complications. This can be mitigated by reducing the curvature of the TSC system while avoiding a superior TSC puncture site.
Congenital Defects Involving the Interatrial Septum
The most common congenital abnormality affecting the IAS is the presence of patent foramen ovale (PFO), which is usually associated with an aneurysmal IAS.18,19 ICE is once again superior to plain fluoroscopy in identifying a PFO, which should be avoided for obtaining access to the LA given that TSC via PFO hinders catheter manipulation in the LA and makes the inferior pulmonary veins especially hard to reach. Longer procedure times have also been reported with TSC via PFO.20 Unintended TSC via PFO is evidenced by leftward displacement of the TSC apparatus without the deployment of the needle at a superior and anterior location over the IAS, which can be easily visualised with ICE. We, therefore, take every step to avoid going across the PFO when performing TSC. The second most common congenital defect affecting the IAS is secundum-type atrial septal defect (ASD), which accounts for 70% of all reported ASDs.21 A secundum ASD is relatively easy to go across because it usually affects the central portion of the fossa ovalis.
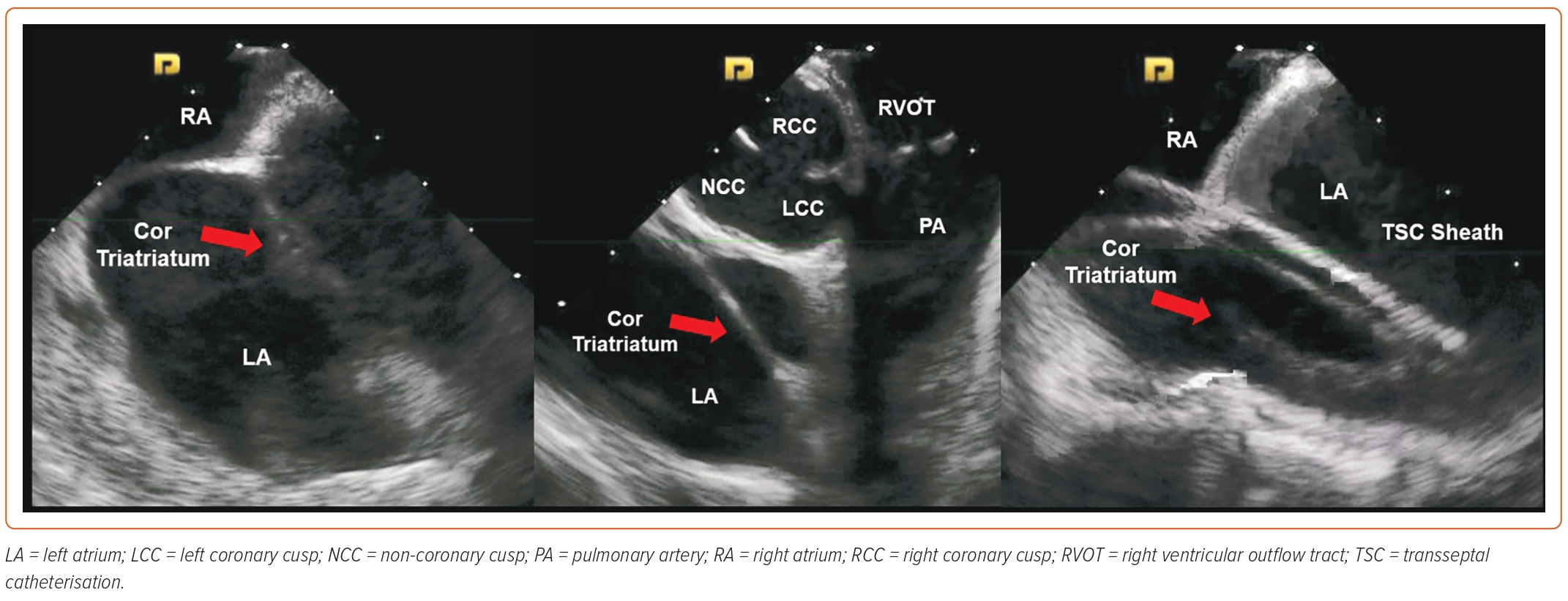
The least common of all congenital abnormalities is the double IAS: a variant of cor triatriatum (Figure 3), which comes with its own set of potential challenges when performing TSC. ICE can be very helpful in such cases given that, quite often, there is a small portion of the IAS that is not divided, and transseptal access at that location avoids any interactions with the interatrial chamber and leads to a lower risk of stroke.22
Interatrial Septal Closure Devices
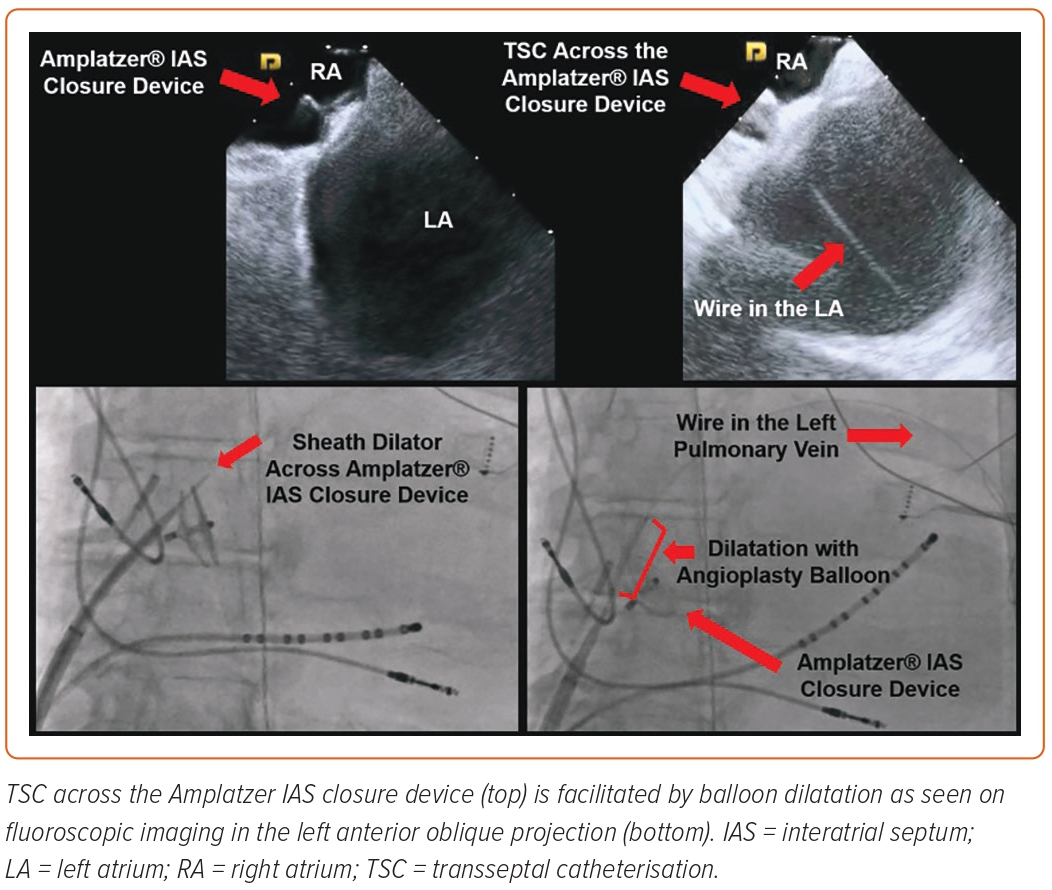
As previously discussed, congenital defects affecting the IAS, although uncommon, can, in certain cases, be symptomatic and lead to pulmonary hypertension, right heart failure and paradoxical stroke. Historically, surgical repair has been used to close these defects but the use of implantable closure devices deployed using a transcatheter approach is currently the preferred closure technique owing to the lower risk profile and healthcare costs (Figure 4).23,24 Classically, the presence of IAS closure devices was deemed to be a contraindication for TSC on account of the risk for device dislodgement, malfunction and abnormal intracardiac shunting. The safety and feasibility of TSC in a series of 39 patients with IAS closure devices was studied by Santangeli et al., who noted that 82% of the patients had an Amplatzer and 18% had a Cardioseal closure device.25 Aided by ICE and fluoroscopy, TSC was performed by crossing the parts of the IAS not covered by the closure device (90%) or by going through the peripheral body of the closure device itself (10%; all Amplatzer devices). All of the patients underwent dual TSC and the devices that were punctured directly were more than 18 months old. The TSC across parts of the native IAS was done with the standard techniques but TSC across the closure device required additional steps. After initial puncture with the needle, a stiff exchange-length guidewire was secured in the LA. Progressive dilatation of the puncture site to an 11 Fr sheath was performed, which established successful access to the LA. Dual TSC access to the LA through the closure device required significantly more time when compared with TSC across the parts of native IAS.25 That study did not show any procedural complications or significant intracardiac shunting at 3- and 6-month follow-ups. Subsequently, Chen et al. reported the case of a patient with an Amplatzer septal closure device for a large secundum ASD who presented for catheter ablation of AF. Successful TSC across the septal occlusion device was performed using a Brockenbrough needle followed by dilatation with an angioplasty balloon.26 Incorporation of preprocedural cardiac imaging, such as multidetector cardiac CT into the 3D mapping systems or integration of ICE with the electroanatomic map, can further help to visualise the IAS closure devices and aid TSC during such procedures.
Inferior Vena Cava Obstruction
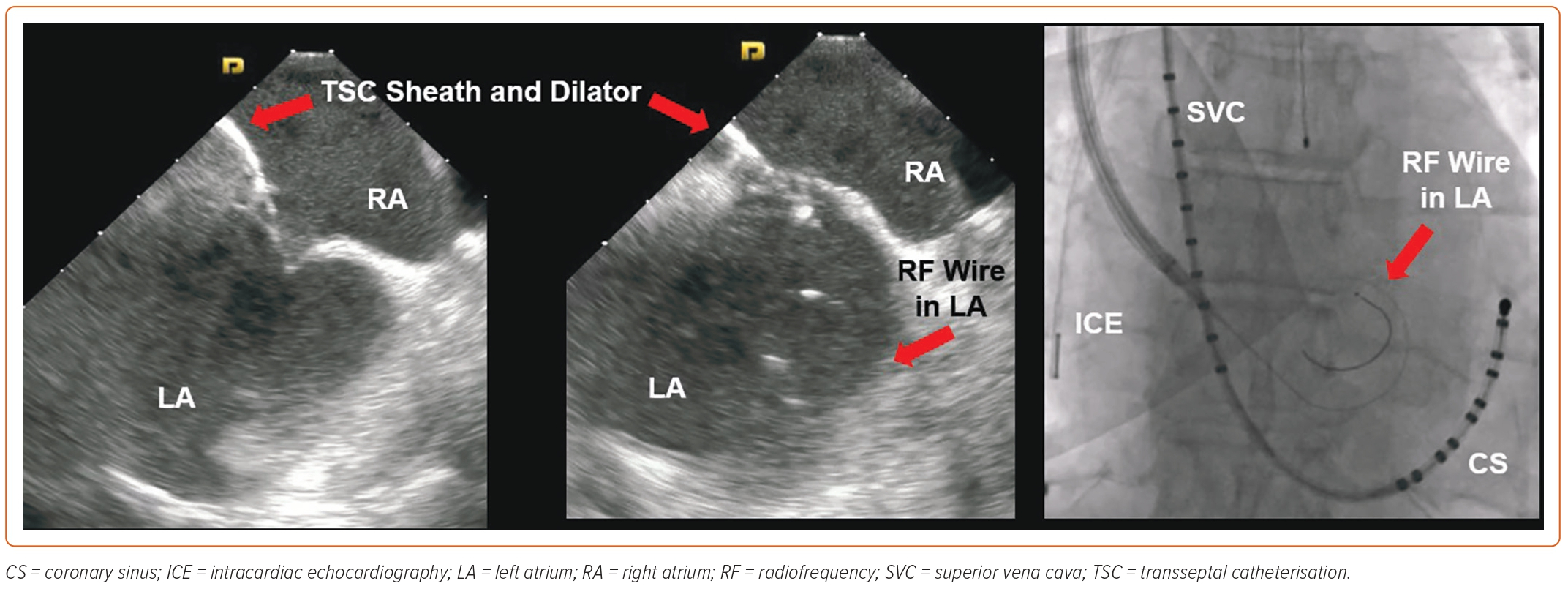
In a rare subset of patients presenting to the electrophysiology lab, inferior vena cava (IVC) is found to have a total occlusion. A vast majority of these patients have had hepatobiliary surgery or hypercoagulability, leading to IVC thrombosis and subsequent total occlusion. Some of these patients have congenitally absent IVC with the continuation of azygous vein distally. One option in such cases is to obtain transhepatic access with the help of ultrasound and fluoroscopic guidance but this requires the presence of an intact suprahepatic IVC and opens the door to potential complications. This has been performed in paediatric patients with complex congenital abnormalities involving the IVC and the superior vena cava.27 A more recent experience with adult patients with interrupted IVC requiring transhepatic access to the suprahepatic IVC was reported by Singh et al., who demonstrated the safety and feasibility of this procedure.28 In our experience, this not only increases the risk of abdominal bleeding but is also cumbersome due to the catheter manipulation during the procedure. We prefer the superior approach to perform TSC in patients with interrupted IVC, given its safety profile and the fact that it is less time consuming. A superior approach for ablating right atrial arrhythmias has been reported by several authors and can be performed from the right internal jugular veins.29–32 The same approach can be used to access the left ventricle for endocardial lead placement and for left atrial ablation with documented safety and feasibility.33–36 Lim et al. reported on a TSC performed via the right internal jugular vein using an 8 Fr fixed curve sheath and Brockenbrough needle that was manually curved to a 150° angle.34 The TSC apparatus was advanced to the RA under fluoroscopy to the level of the ostium of the coronary sinus. With the needle inserted, the system was gently pulled back with counterclockwise rotation to engage the fossa ovalis. Beyond that, standard manoeuvres were carried out to perform TSC.34 In our practice, we have moved on to a right axillary vein access instead of using the right internal jugular vein, as described in a recent publication.37 This makes the catheter manipulation less cumbersome, and we use ICE and fluoroscopic guidance in these cases. We prefer using a pigtail guidewire with RF capability instead of a needle to go across the IAS, which improves the safety profile and manoeuvrability of the sheath during TSC. With this approach, a steerable sheath is advanced to the RA over a guidewire and the dilator is pulled back into the sheath. The sheath is rotated in a counterclockwise manner and deflected to engage the IAS as visualised on ICE. Using RF energy applied from the tip of the guidewire, the IAS is crossed, and the guidewire is advanced into the LA, over which the dilator and the sheath are advanced as well. We typically perform single transseptal access in such cases followed by standard LA mapping and ablation (Figure 5). An important point to remember is that the movements of the sheath are reversed with this approach.
Surgically Repaired Congenital Heart Disease
A unique challenge is posed by patients with complex congenital heart disease that has been surgically repaired. These patients are prone to arrhythmias and abnormalities of the sinus node and conduction system, requiring a plethora of electrophysiological procedures.38 Many of these patients develop atrial ventricular arrhythmias secondary to the underlying anatomy and the subsequent surgical procedures that they undergo, and require a multidisciplinary approach. Preprocedural planning to obtain old records, including surgeries, cardiac interventions and cardiovascular imaging, is mandatory. Contemporary 3D imaging to ascertain the current cardiovascular anatomy is important, given that this will guide procedural planning. The most important of the surgically corrected congenital abnormalities that hinder access to the atria are dextro-transposition of the great arteries after Mustard or Senning repair, and patients with Fontan repair. In both of these conditions, parts of the atria are separated from the venous circulation, and the best route to perform mapping and ablation is to go across the surgical grafts. These procedures require fluoroscopy, ICE and/or TOE guidance throughout the procedure and can be performed safely. There are a few publications that have addressed these challenges and have provided a step-by-step approach to circumvent them.38,39 In summary, these patients can have sinus node dysfunction, atrial arrhythmias, such as intra-atrial re-entrant tachycardia, supraventricular tachycardia using accessory bypass tracts and ventricular tachycardia, that can lead to sudden death. Several techniques discussed above can be used to obtain access to areas of the heart inaccessible due to the altered anatomy and surgical palliation.
Conclusion
Transseptal catheterisation is an important skill for cardiac proceduralists and it can be performed safely in a wide range of patients, including the ones with specific challenges. A number of procedural steps can be undertaken to successfully perform TSC in such challenging cases while maintaining a high safety profile. These techniques can be used to perform diagnostic and therapeutic electrophysiological procedures for atrial or ventricular arrhythmias. In these cases, we strongly recommend detailed preprocedural planning and the use of real-time imaging with ICE. 
Clinical Perspective
- With an increasing number of electrophysiological procedures, we frequently run into cases of challenging atrial transseptal catheterisation.
- Preprocedure planning and intracardiac echocardiography are of paramount importance in performing these procedures successfully.
- With the current tools available to us, we can safely perform atrial transseptal catheterisation in patients in whom it was previously considered contraindicated.