Ventricular conduction disturbances are common in patients with heart failure. They result in loss of synchronous electrical activation and ventricular contraction.1 Despite the demonstrated benefits of biventricular pacing (BiVP), cardiac resynchronisation via BiVP remains suboptimal due to the non-physiologic fusion of paced wavefronts, and approximately 30% of BiVP recipients do not have an improvement in heart failure.2–4 Disruption of cardiac depolarisation also results in potentially abnormal repolarisation, which can increase the risk of ventricular arrhythmia and sudden cardiac death.5
Conduction system pacing (CSP) has emerged as an alternative to BiVP, and this includes pacing at either the His bundle (HB) or left bundle locations. In 2000, the clinical feasibility of His bundle pacing (HBP) was first reported by Deshmukh et al. in patients with chronic AF and left ventricular (LV) dysfunction.6 To overcome some of the inherent limitations of HBP, left bundle branch area pacing (LBBAP) was first reported in 2017 by Huang et al. and has been increasingly adopted over time.7
Due to the recruitment of the native conduction system, CSP is more physiological than currently available standard BiVP and can potentially improve clinical outcomes in patients with heart failure.8 The objective of this review is to summarise the current options and outcomes of CSP when used in patients eligible for cardiac resynchronisation therapy (CRT).
Options for Cardiac Resynchronisation Therapy Using Conduction System Pacing
His Bundle Pacing
Anatomy
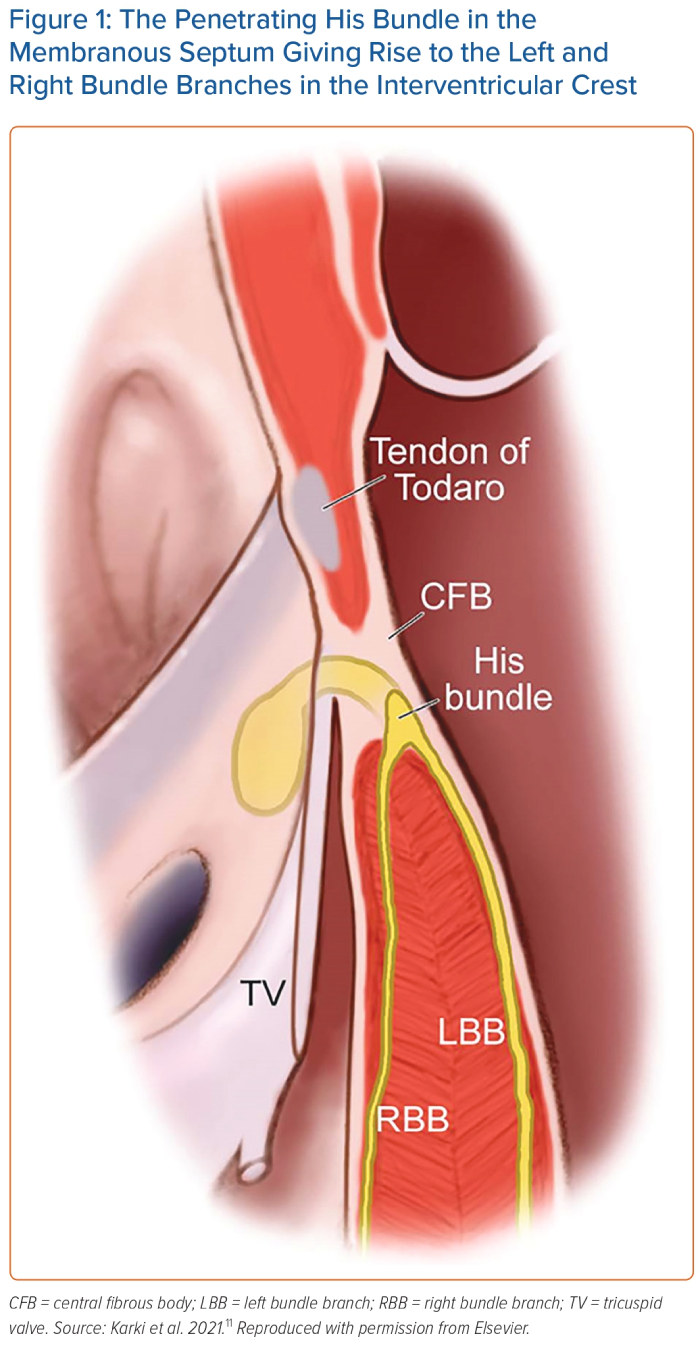
The HB is a specialised muscle bundle that electrically connects the atria and ventricles. It was discovered by Wilhelm His Jr in 1893 and was further characterised by Tawara in 1906.9,10 The HB can be divided into a penetrating portion, a non-branching portion, and a branching portion. The mean dimensions of the penetrating portion are approximately 2.6 mm (length) × 3.7 mm (width) × 1.4 mm (thickness).9 The HB resides in the membranous septum, which is divided by the septal leaflet of the tricuspid valve (TV) into two portions: an atrioventricular portion and a ventriculoventricular portion.11 It usually runs along the left side of the membranous septum and bifurcates into the right and left bundle branches at the interventricular crest (Figure 1). The HB is encapsulated and electrically insulated by the central fibrous body.
Mechanisms of Paced QRS Narrowing
HBP is achieved by implanting a permanent pacing lead into the HB to recruit the native conduction system and restore ventricular synchrony. Potential mechanisms for QRS narrowing with HBP in patients with typical bundle branch blocks include virtual electrode polarisation stimulation distal to the block, transverse bundle connections, source–sink mismatch effects, and longitudinal dissociation of predestined fibres within the HB. In patients without intra-Hisian disease, the underlying cardiomyopathy may still affect the transverse interconnections within the HB and set up a functional block that can be overcome by HBP.12
Available Tools
Pacing Lead
The SelectSecure 3830 lead from Medtronic (69 cm long solid core, 4.1 Fr (1.4 mm) body diameter, bipolar, lumenless, 1.8 mm fixed exposed helix screw, active fixation) is approved by the Food and Drug Administration for CSP.13–15 Other leads are being investigated as well.13
Delivery Sheaths
There are multiple sheath options for HBP, each with particular advantages.13 Two commonly used sheaths are worth mentioning:
- C315His sheath from Medtronic (non-deflectable, 5.4 Fr inner diameter and 7.0 Fr outer diameter, 43 cm long, with a primary curve to reach the TV annulus, and a secondary curve to reach the septum).14,15
- SelectSite C304-His sheath from Medtronic (deflectable, 5.7 Fr inner diameter and 8.4 Fr outer diameter, 43 cm long, with unidirectional deflection), helpful for intraprocedural anatomic challenges.14,15
Landmarks for Lead Placement
The main anatomical landmark to locate the HB is the membranous septum at the level of the TV annulus.10 Visualisation of the TV annulus via contrast injection through a delivery sheath can be performed before mapping.16 The penetrating HB can be above, at the level or below the level of the TV hinge point.9 Either the atrial or the ventricular part of the membranous septum can be targeted for HBP; however, the ventricular part is usually preferred to avoid atrial or His oversensing and/or ventricular undersensing.17 A guidewire is positioned in the right ventricular outflow tract, and the delivery sheath is advanced over the wire into the right ventricle (RV). The delivery sheath is then anchored with good contact on the ventricular side of the TV annulus. The lead is advanced through the sheath for mapping. Once an HB potential is localised, pacing is attempted to confirm HB capture. After identifying an optimal site for HBP, the pacing lead is then screwed.14,15
Technical Details for Lead Placement
Using subclavian or axillary venous access to the right atrium, a counter-clockwise torque is needed to reach the septum by the TV annulus. The C315His sheath (Medtronic) has been the preferred sheath for implanting an HBP lead. The secondary septal curve enables the lead tip to be securely fixed due to a perpendicular orientation of the lead tip to the myocardial surface. The SelectSite C304-His sheath (Medtronic) is useful for challenging anatomies but lead fixation with this sheath can be more difficult.13–15 Right atrial enlargement and severe tricuspid regurgitation can make HBP lead implantation difficult.18
Criteria for Optimal His Bundle Pacing
In general, optimal parameters for HBP include a sensed R wave of >1.5–2 mV without atrial oversensing and a unipolar pacing threshold of <1.5 V at a pulse width of 1 ms.14,15 Upon fixation of the pacing lead, acute HB injury current is considered a prognostic factor for lower and durable pacing thresholds in the long term.19 Occasionally, far-field non-ventricular electrograms can be oversensed on the ventricular channel, which can inhibit ventricular pacing.
HBP can be achieved in two ways: selective HBP (S-HBP) and non-selective HBP (NS-HBP).20 The criteria for S-HBP are: a stimulus–ventricle interval (S-V) equal in duration to the intrinsic His–ventricle interval (H-V); and a paced QRS morphology identical to the intrinsic QRS complex. Criteria for NS-HBP are: a shorter S-V than the intrinsic H-V; and local fusion of both the HB and septal myocardium signals.14,15 Both pacing modalities have been reported to have similar outcomes as long as both bundle branches are recruited (left and right).20,21
Success Rate
In a systematic review of a total of 13 studies involving 503 patients undergoing HBP for cardiac resynchronisation, an average implant success rate of 79.8% was reported.22 A trend of increase in capture thresholds was observed at follow-up.22 In high-risk patients, an RV lead can be implanted for back-up pacing. The presence of proximal conduction system block is a useful predictor of successful ventricular resynchronisation using HBP. Upadhyay et al. found, using intracardiac septal mapping, that conduction block in the left-sided His fibres or proximal part of the left bundle branch (LBB) was present in 64% of patients with left bundle branch block (LBBB).23 These patients have the highest likelihood of successful resynchronisation with HBP.23
Factors for Failure
Atrial oversensing, ventricular undersensing, unstable lead fixation and elevated pacing thresholds are all factors associated with HBP failure.24 The success of HBP is also adversely impacted by infra-Hisian conduction impairment due to its inability to correct distal conduction system disease.25,26
Complications
HBP lead-related complications include lead dislodgement and progressive increases in capture thresholds. In a multicentre study by Zanon et al. assessing the long-term performance and safety of HBP, HBP was free of any complication in 91.6% of patients after a median follow-up of 3 years.27 Better pacing and sensing parameters were noted with the fixed curve C315 sheath than with the deflectable curve C304 sheath.27 In a 5-year follow-up study by Vijayaraman et al., approximately 12% of patients undergoing HBP had an increase in pacing thresholds.24 The unacceptably high pacing thresholds ultimately resulted in a higher rate of lead revision.28
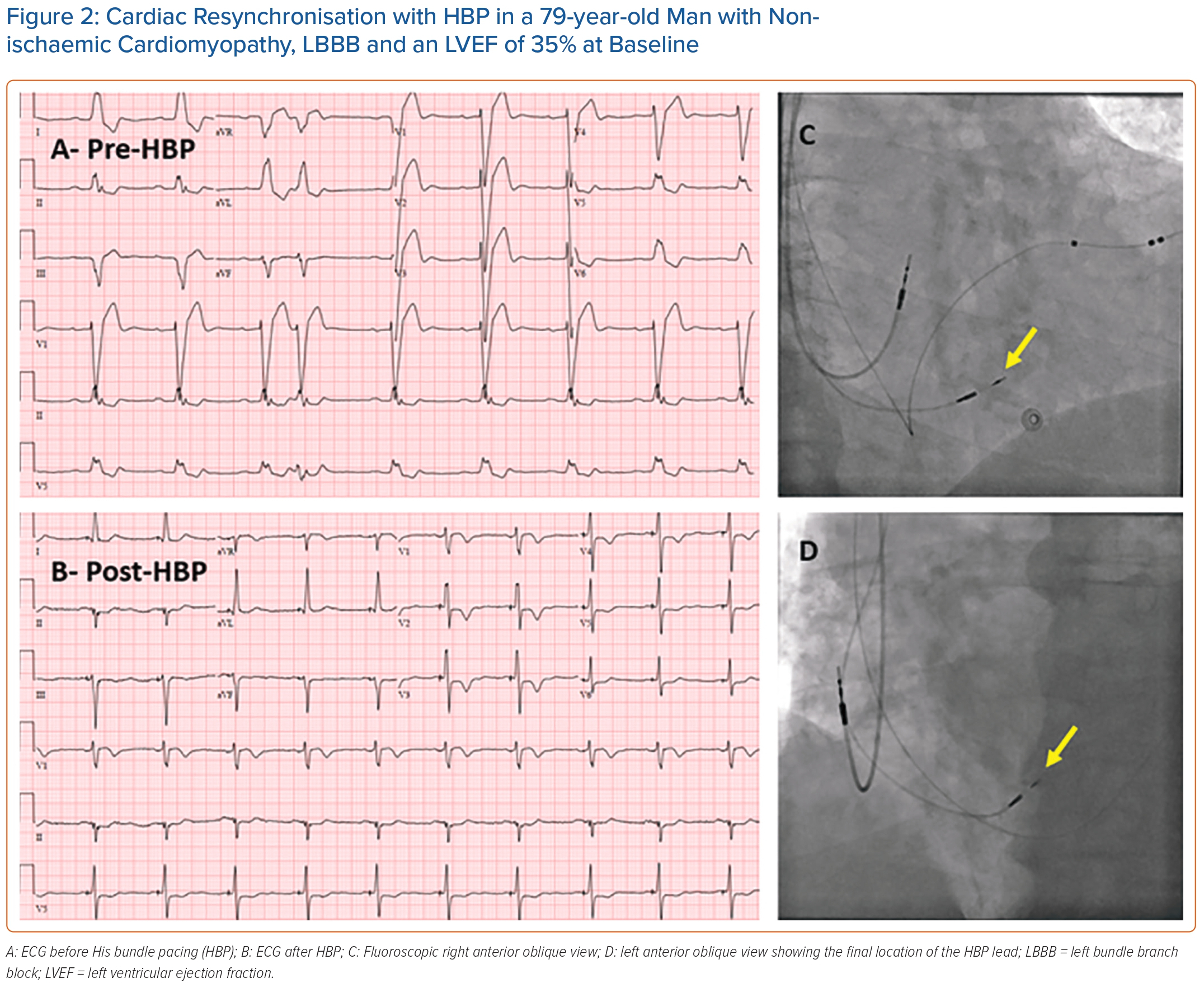
Case Example
Figure 2 shows an example of successful cardiac resynchronisation with HBP in a patient with non-ischaemic cardiomyopathy, LBBB and a depressed left ventricular ejection fraction (LVEF) at baseline. QRS duration narrowed from 194 ms before HBP to 110 ms after HBP. Three months after HBP, LVEF improved from 35% before HBP to 50% after HBP.
Left Bundle Branch Area Pacing
Anatomy
LBBAP is another form of CSP that has the potential to overcome the limitations associated with HBP by targeting the region below the HB.29,30 The LBB is a band-like structure that is thicker than the HB, encased in fibrous tissue and surrounded by dense muscular tissue (Figure 1). Despite the marked variability in the anatomy of the LBB, histopathological investigations have shown that the LBB arborises into three distal networks: a thin and elongated anterior fascicle, a wider posterior fascicle and a midseptal fascicle. The proximal LBB is the main target for LBBAP. However, due to the anatomical variability in the branching pattern of the LBB, the pacing lead may target the septal fascicle or even the posterior one.
Mechanisms of Paced QRS Narrowing
In patients with LBBB, conduction slowing or block within the LBB can be bypassed with LBBAP by pacing distal to the block. The strength–duration curve of the LBB is shifted to the left of the myocardium strength–duration curve and can be captured from a distance by optimising the pulse width and maximising the virtual electrode effect.31 Therefore, precise positioning of the pacing lead in the LV subendocardium is not mandatory in LBBAP to capture the left bundle system efficiently. In patients with right bundle branch block (RBBB), potential mechanisms for QRS narrowing with LBBAP include capturing the right bundle branch through transverse interconnections or exciting the right septal myocardium by anodal capture of bipolar pacing.32
Landmarks for Lead Placement
LBBAP has a wider and more predictable target zone for pacing than HBP. The LBB is located around 1.5–2 cm distal to the HB. The TV annulus is used as a point of reference.
Available Tools
The same lead and sheaths used for HBP can also be used for LBBAP (the 3830 SelectSecure lead, and the C315His or SelectSite C304-His sheaths [Medtronic]).33 Multiple vendors have also introduced delivery sheaths for CSP using standard stylet-driven leads, which have been gaining popularity.34
Technical Details for Lead Placement
Signs of good potential sites for LBBAP are paced QS morphology in lead V1 (with or without a notch), QRS discordance in the inferior leads (predominantly positive in lead II and biphasic or negative in lead III), and QRS discordance in lead aVR/aVL (negative in aVR and positive in aVL). Once this paced QRS morphology is identified, the septum is penetrated and the lead is screwed with 3–5 clockwise lead turns. Ideal septal lead depth is around 1.4 ± 0.23 cm.35
Monitoring fixation beats, which are ectopic beats with qR/rsR′ morphology in lead V1, can help predict whether the desired septal depth was reached during lead deployment.36 If lead placement is unsuccessful, replacement of the sheath or repositioning of the lead more distally and more inferiorly can be considered. A 22–24 G needle can be used to remove the tissue lodged in the helix.
Criteria for Optimal Left Bundle Branch Area Pacing
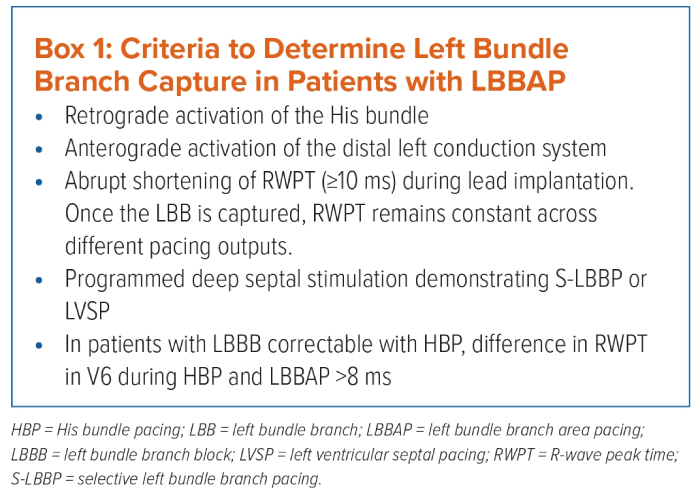
Left bundle branch pacing (LBBP) is defined as capture of the LBB, usually with septal myocardium capture at a low pacing output (<1 V at 0.4 ms).37 Box 1 summarises the criteria that have been described to confirm LBB capture during LBBAP:
- Peak left ventricular activation time (pLVAT): it remains short (<80 ms) and stable with LBB capture across different pacing outputs.37 In patients with LBBB correctable with HBP, an absolute value of 8 ms for the difference in R wave peak times (RWPT) in the lateral precordial leads during HBP and LBBAP confirms LBB capture.38
- LBB potential: it is a sharp high-frequency deflection 15–30 ms before the onset of surface QRS.39
- Retrograde His potential and anterograde distal LBB potentials.40
- Selective versus non-selective LBBP: prolonged pLVAT when the output is changed from high to low, longer stimulus–His interval and longer stimulus–right atrial interval are all indicators of non-selective LBBP.40
- Programmed stimulation: it is useful for differentiating septal and LBB capture.41
Programmed deep septal stimulation can be performed if LBB capture is equivocal.41 Unlike HBP, it is more difficult to discern recruitment of the conduction system in LBBAP by simply changing the pacing output because the pacing thresholds of the septal myocardium and LBB are similar. The septal myocardial refractory period is shorter than the LBB refractory period, which makes programmed deep septal stimulation useful for confirming LBB capture. Interestingly, programmed deep septal stimulation shows that direct LBB capture can be present despite a wide-paced QRS complex, lack of LBB potential on the pacing lead electrogram and even lack of r′ in lead V1 QRS.41
Success Rate
In a systematic review and meta-analysis assessing the safety and efficacy of LBBAP in patients with heart failure and LBBB, a total of six studies involving 177 patients were analysed.42 The average implant success rate was 93.2%.42 LBBAP resulted in significant narrowing of QRS duration and improvement in LVEF, left ventricular end diastolic diameter and New York Heart Association (NYHA) functional class.42 The incidence of complications was 2.4%.42 Given the wide area of LBB targeted with LBBAP, LBBAP thresholds are stable over time.35,39,43,44 Even though, theoretically, LBBAP may potentially delay RV activation resulting in loss of RV synchrony, this has not been an issue, probably due to myocardial septal capture, fusion with native right bundle conduction and/or retrograde conduction from the left bundle to the right bundle.
Factors of Failure
Factors that may cause failure to advance the pacing lead into the septum include inadequate sheath support or incorrect sheath orientation, septal scar and/or fibrosis, tissue lodging into the helix, and deformed sheath or helix. LBBAP lead implantation failure is more likely to occur in patients with heart failure, enlarged left ventricle and prolonged QRS duration.45
Complications
Potential complications of LBBAP include septal perforation, thromboembolism, right bundle branch injury, septal arterial injury and lead dislodgement.33 The most common complication of LBBAP is septal perforation, which can be suspected when there is decrease in the sensed R wave amplitude, increase in the capture threshold and/or decrease in the unipolar impedance below 500 Ω.33 Evaluating the basal interventricular septum thickness and lead length can help prevent septal perforation.
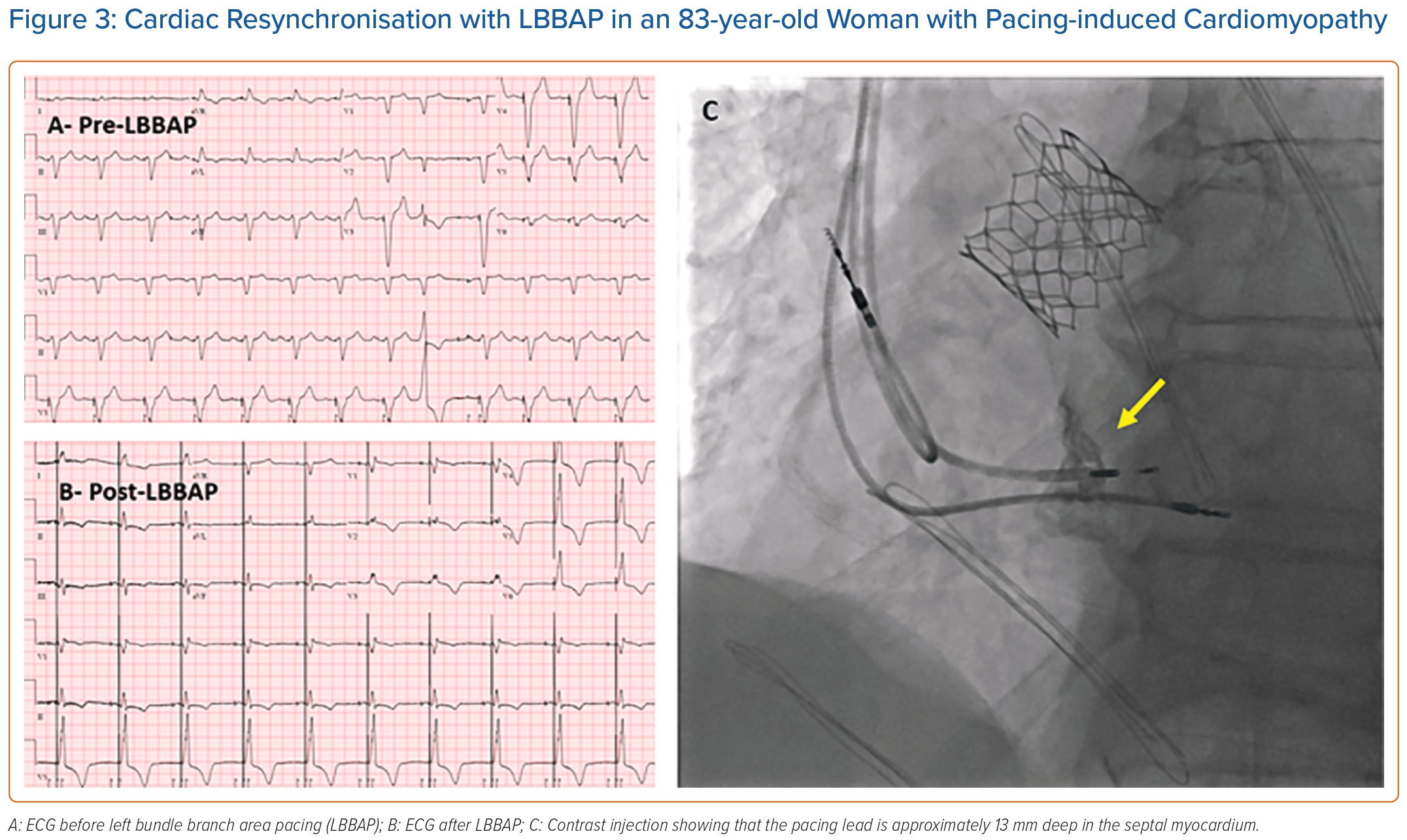
Case Example
Figure 3 shows an example of successful cardiac resynchronisation with LBBAP in an 83-year-old woman with pacing-induced cardiomyopathy. The 3830 SelectSecure (Medtronic) lead was screwed in an RV basal inferior location. Paced RBBB morphology was obtained with an R wave peak time in lead V6 of 80 ms in duration. The paced QRS duration was 130 ms. Contrast injection showed that the lead was 13 mm deep into the septal myocardium. Three months after LBBAP, LVEF improved from 29% before LBBAP to 35% after LBBAP.
Hybrid Conduction System Pacing Methods
Hybrid CSP methods for CRT rely on using an LV epicardial lead in addition to an HB lead or LBB area lead. They are referred to as His-optimised CRT (HOT-CRT) and LBB-optimised CRT (LOT-CRT).46–48 These hybrid methods of pacing appear to be promising, particularly in patients with mixed conduction disease and myocardial delay or conduction block at multiple levels.
Outcomes of Conduction System Pacing for Cardiac Resynchronisation
Conduction System Pacing versus Biventricular Pacing
BiVP is the standard pacing modality for cardiac resynchronisation in patients with heart failure, which is recommended by current guidelines.49 However, up to one-third of all CRT candidates do not respond to BiVP.50 A growing body of evidence has demonstrated the feasibility and efficacy of CSP as an alternative to BiVP in CRT candidates. Vijayaraman et al. conducted an observational study comparing CSP and BiVP in 477 patients eligible for CRT.51 CSP resulted in greater improvement of LVEF than BiVP during a mean follow-up of 27 months.51 The primary outcome of death or heart failure hospitalisation was significantly lower with CSP compared with BiVP.51 The difference in clinical outcomes was more pronounced in patients with LBBB.51 In a multicentre observational study of 238 patients eligible for CRT, Ezzeddine et al. demonstrated a greater rate of CRT response with CSP than with BiVP in patients with heart failure with reduced ejection fraction.52 The clinical outcomes of death and heart failure hospitalisation were similar between the two groups.52
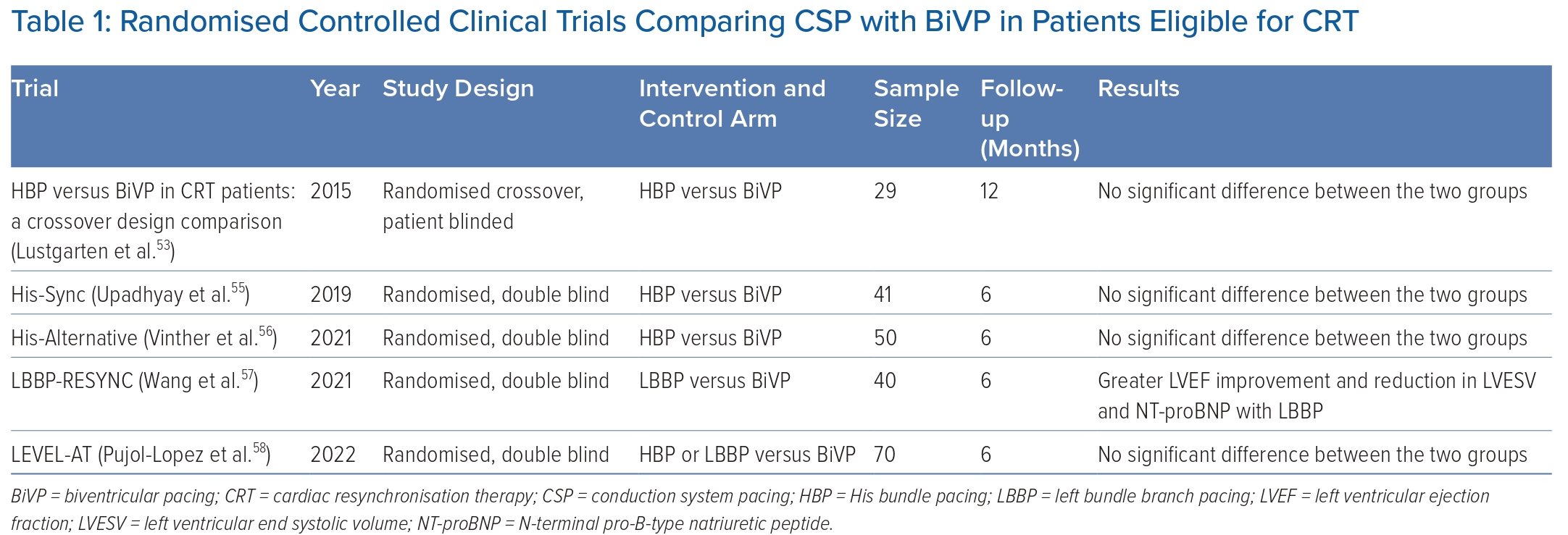
Table 1 lists the clinical trials comparing the clinical efficacy of CSP and BiVP that have been completed and published to date. The first randomised controlled clinical trial comparing the clinical response of HBP with BiVP in CRT-eligible patients was published in 2015 by Lustgarten et al.53 In that study, clinical outcomes were significantly better as compared with baseline for both HBP and BiVP.53 With HBP, QRS narrowing was noted in the majority of patients with ischaemic cardiomyopathy but in only half of the patients with non-ischaemic cardiomyopathy.53 One possible explanation could be the presence of more proximal lesions in patients with ischaemic cardiomyopathy causing bundle branch disease, which can be bypassed by CSP.
The His-Sync trial is the first randomised pilot trial comparing the feasibility and efficacy of HBP with that of BiVP. No significant differences in ECG and echocardiogram parameters were noted between HBP and BiVP.54 The study, however, was underpowered to detect differences <10% between the two groups.54 It was also limited by high cross-over rates between the two groups.54 In a secondary analysis of the trial, patients receiving HBP had better electrical resynchronisation and a trend toward better echocardiographic response than patients receiving BiVP.55
Given that patients with intraventricular conduction delay are less likely to respond to HBP, the His Alternative clinical trial was designed to compare the feasibility and efficacy of HBP with BiVP in patients with complete LBBB.56 The study demonstrated similar clinical improvement with HBP as compared with BiVP at the expense of higher pacing thresholds.56 The per-protocol LVEF was significantly higher, and the left ventricular end systolic volume (LVESV) was significantly lower at 6 months in patients receiving HBP than in patients receiving BiVP.56
The LBBP-RESYNC trial is the first randomised controlled clinical trial comparing LBBAP with BiVP.57 The study included patients with non-ischaemic cardiomyopathy and complete LBBB. Intention-to-treat analysis showed significantly greater LVEF improvement and reductions in LVESV and N-terminal pro-B-type natriuretic peptide (NT-proBNP) at 6 months with LBBP than with BiVP.57
The LEVEL-AT trial is the first randomised controlled clinical trial comparing LVAT between CSP and BiVP.58 In this study, patients eligible for CRT were randomised to receive CSP (HBP or LBBAP) or BiVP. CSP resulted in a similar cardiac resynchronisation to that with BiVP, with similar changes in LVAT and QRS duration.58 Comparable improvement in NYHA functional class and 6-month rates of heart failure hospitalisation and mortality were also noted between the two groups of CSP and BiVP.58
In summary, these trials demonstrated non-inferior or even greater electrical synchronisation with CSP as compared with BiVP in patients with heart failure who are eligible for CRT. However, data on long-term clinical outcomes of CSP in these patients remain limited.
Conduction System Pacing in Patients with Right Bundle Branch Block
Patients with heart failure and RBBB have been shown to have limited benefit from conventional BiVP.59,60 When concomitant LV activation delay is present, patients with RBBB derive more benefit from BiVP. In patients with RBBB only, CSP is a viable option for restoring ventricular synchronisation by correcting the RBBB and preserving physiologic LV activation. In a multicentre observational study by Sharma et al., HBP resulted in significant QRS narrowing and improvement in LV function in patients with heart failure, RBBB and reduced LVEF.61 HBP is more effective than BiVP in patients with RBBB and conduction slowing affecting the whole His–Purkinje system or ventricular myocardium.62
In a multicentre observational study by Vijayaraman et al., LBBAP was also effective at narrowing QRS duration and improving LV function in patients with heart failure, RBBB and reduced LVEF.63 Female sex and reduction in QRS duration were predictive of echocardiographic CRT response and super-response.63 RV synchrony is improved with LBBAP when it is delivered in combination with anodal capture of the RV septum.62
Conduction System Pacing in Patients with Atrioventricular Node Ablation
In patients with AF and high ventricular rates that are refractory to other treatment modalities, atrioventricular node ablation (AVNA) with permanent pacemaker implantation is an effective treatment approach.64,65 The APAF-CRT trial demonstrated a significant reduction in mortality in patients with heart failure and a narrow QRS duration who were randomised to AVNA and CRT compared with pharmacological therapy.66 In that patient population, CSP with either HBP or LBBAP was safe, feasible and associated with improvement in LV function.67–69 However, AVNA with LBBAP pacing is associated with a higher success rate and fewer acute and chronic lead-related complications.70
In a retrospective observational study, Vijayaraman et al. compared the procedural and clinical outcomes of CSP versus BiVP in 223 patients undergoing AVNA and CRT.71 CSP was associated with a reduction in the primary outcome of death and heart failure hospitalisation compared with BiVP, although this was likely to be due to a sicker group of patients in the BiVP group with a lower LVEF and wider QRS at baseline.71
Conclusion
CSP is a physiological pacing modality that has been shown to improve clinical, ECG and echocardiographic outcomes in patients with heart failure by providing effective electromechanical ventricular synchronisation. The outcomes of HBP versus LBBAP may be variable depending on the patient population in which they are performed. Large-scale prospective randomised trials are needed to compare the long-term outcomes of CSP and BiVP. 
Clinical Perspective
- Conduction system pacing (CSP) is a promising option for cardiac resynchronisation therapy because it restores physiologic ventricular synchrony by recruitment of the native conduction system.
- His bundle pacing (HBP) is limited by high and often unstable pacing thresholds, high rates of lead revision, and inability to correct distal conduction system disease.
- Compared with HBP, left bundle branch area pacing has stable pacing thresholds and a higher likelihood of correcting distal conduction system disease.
- Hybrid CSP methods (CSP and coronary sinus pacing) are particularly beneficial in patients with mixed conduction disease and myocardial delay or conduction block at multiple levels.